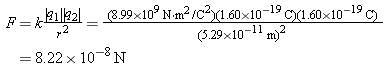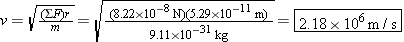|
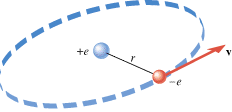
In the Bohr model of the hydrogen atom, the electron (–e) is in orbit about the nuclear proton (+e) at a radius of r = = 5.29 5.29 × × 10–11 m, as Figure 18.11 shows. Determine the speed of the electron, assuming the orbit to be circular. 10–11 m, as Figure 18.11 shows. Determine the speed of the electron, assuming the orbit to be circular.
 | Figure 18.11
In the Bohr model of the hydrogen atom, the electron (–
e) orbits the proton (+
e) at a distance of r
 =
=
 5.29 5.29 ×
×
 10
–11 m. The velocity of the electron is v. 10
–11 m. The velocity of the electron is v. |
|
Reasoning
Recall from Section 5.2 that any object moving with speed v on a circular path of radius r has a centripetal acceleration of ac = = v2/r. This acceleration is directed toward the center of the circle. Newton’s second law specifies that the net force SF needed to create this acceleration is SF v2/r. This acceleration is directed toward the center of the circle. Newton’s second law specifies that the net force SF needed to create this acceleration is SF = = mac mac = = mv2/r, where m is the mass of the object. This equation can be solved for the speed: mv2/r, where m is the mass of the object. This equation can be solved for the speed: 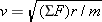 . Since the mass of the electron is m . Since the mass of the electron is m = = 9.11 9.11 × × –31 kg and the radius is given, we can calculate the speed, once a value for the net force is available. For the electron in the hydrogen atom, the net force is provided almost exclusively by the electrostatic force, as given by Coulomb’s law. This force points toward the center of the circle, since the electron and the proton have opposite signs. The electron is also pulled toward the proton by the gravitational force. However, the gravitational force is negligible in comparison to the electrostatic force. –31 kg and the radius is given, we can calculate the speed, once a value for the net force is available. For the electron in the hydrogen atom, the net force is provided almost exclusively by the electrostatic force, as given by Coulomb’s law. This force points toward the center of the circle, since the electron and the proton have opposite signs. The electron is also pulled toward the proton by the gravitational force. However, the gravitational force is negligible in comparison to the electrostatic force.
Problem solving insight
When using Coulomb’s law (F = = k |q1||q2|/r2), remember that the symbols |q1| and |q2| stand for the charge magnitudes. Do not substitute negative numbers for these symbols. k |q1||q2|/r2), remember that the symbols |q1| and |q2| stand for the charge magnitudes. Do not substitute negative numbers for these symbols. |
|
Solution
The electron experiences an electrostatic force of attraction because of the proton, and the magnitude of this force is
Using this value for the net force, we find
This orbital speed is almost five million miles per hour.
|
![]() =
=![]() 5.29
5.29![]() ×
×![]() 10–11 m, as Figure 18.11 shows. Determine the speed of the electron, assuming the orbit to be circular.
10–11 m, as Figure 18.11 shows. Determine the speed of the electron, assuming the orbit to be circular.
![]() =
=![]() v2/r. This acceleration is directed toward the center of the circle. Newton’s second law specifies that the net force SF needed to create this acceleration is SF
v2/r. This acceleration is directed toward the center of the circle. Newton’s second law specifies that the net force SF needed to create this acceleration is SF![]() =
=![]() mac
mac![]() =
=![]() mv2/r, where m is the mass of the object. This equation can be solved for the speed:
mv2/r, where m is the mass of the object. This equation can be solved for the speed: 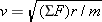 . Since the mass of the electron is m
. Since the mass of the electron is m![]() =
=![]() 9.11
9.11![]() ×
×![]() –31 kg and the radius is given, we can calculate the speed, once a value for the net force is available. For the electron in the hydrogen atom, the net force is provided almost exclusively by the electrostatic force, as given by Coulomb’s law. This force points toward the center of the circle, since the electron and the proton have opposite signs. The electron is also pulled toward the proton by the gravitational force. However, the gravitational force is negligible in comparison to the electrostatic force.
–31 kg and the radius is given, we can calculate the speed, once a value for the net force is available. For the electron in the hydrogen atom, the net force is provided almost exclusively by the electrostatic force, as given by Coulomb’s law. This force points toward the center of the circle, since the electron and the proton have opposite signs. The electron is also pulled toward the proton by the gravitational force. However, the gravitational force is negligible in comparison to the electrostatic force.
![]() =
=![]() k |q1||q2|/r2), remember that the symbols |q1| and |q2| stand for the charge magnitudes. Do not substitute negative numbers for these symbols.
k |q1||q2|/r2), remember that the symbols |q1| and |q2| stand for the charge magnitudes. Do not substitute negative numbers for these symbols.