18.1. The Origin of Electricity
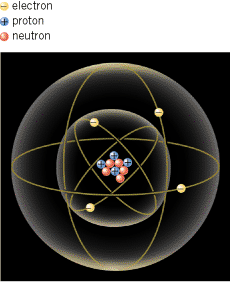
The electrical nature of matter is inherent in atomic structure. An atom consists of a small, relatively massive nucleus that contains particles called protons and neutrons. A proton has a mass of 1.673![]() ×
×![]() 10–27 kg, and a neutron has a slightly greater mass of 1.675
10–27 kg, and a neutron has a slightly greater mass of 1.675![]() ×
×![]() 10–27 kg. Surrounding the nucleus is a diffuse cloud of orbiting particles called electrons, as Figure 18.1 suggests. An electron has a mass of 9.11
10–27 kg. Surrounding the nucleus is a diffuse cloud of orbiting particles called electrons, as Figure 18.1 suggests. An electron has a mass of 9.11![]() ×
×![]() 10–31 kg. Like mass, electric charge is an intrinsic property of protons and electrons, and only two types of charge have been discovered, positive and negative. A proton has a positive charge, and an electron has a negative charge. A neutron has no net electric charge.
10–31 kg. Like mass, electric charge is an intrinsic property of protons and electrons, and only two types of charge have been discovered, positive and negative. A proton has a positive charge, and an electron has a negative charge. A neutron has no net electric charge.
 |
|
Experiment reveals that the magnitude of the charge on the proton exactly equals the magnitude of the charge on the electron; the proton carries a charge +e, and the electron carries a charge –e. The SI unit for measuring the magnitude of an electric charge is the coulomb (C), and e has been determined experimentally to have the value
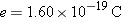 |
The symbol e represents only the magnitude of the charge on a proton or an electron and does not include the algebraic sign that indicates whether the charge is positive or negative. In nature, atoms are normally found with equal numbers of protons and electrons. Usually, then, an atom carries no net charge because the algebraic sum of the positive charge of the nucleus and the negative charge of the electrons is zero. When an atom, or any object, carries no net charge, the object is said to be electrically neutral. The neutrons in the nucleus are electrically neutral particles.
The charge on an electron or a proton is the smallest amount of free charge that has been discovered. Charges of larger magnitude are built up on an object by adding or removing electrons. Thus, any charge of magnitude q is an integer multiple of e; that is, q![]() =
=![]() Ne, where N is an integer. Because any electric charge q occurs in integer multiples of elementary, indivisible charges of magnitude e, electric charge is said to be quantized. Example 1 emphasizes the quantized nature of electric charge.
Ne, where N is an integer. Because any electric charge q occurs in integer multiples of elementary, indivisible charges of magnitude e, electric charge is said to be quantized. Example 1 emphasizes the quantized nature of electric charge.
| Example 1 A Lot of Electrons |