18.2.
Charged Objects and the Electric Force
Electricity has many useful applications, and they are related to the fact that it is possible to transfer electric charge from one object to another. Usually electrons are transferred, and the body that gains electrons acquires an excess of negative charge. The body that loses electrons has an excess of positive charge. Such separation of charge occurs often when two unlike materials are rubbed together. For example, when an ebonite (hard, black rubber) rod is rubbed against animal fur, some of the electrons from atoms of the fur are transferred to the rod. The ebonite becomes negatively charged, and the fur becomes positively charged, as Figure 18.2 indicates. Similarly, if a glass rod is rubbed with a silk cloth, some of the electrons are removed from the atoms of the glass and deposited on the silk, leaving the silk negatively charged and the glass positively charged. There are many familiar examples of charge separation, as when you walk across a nylon rug or run a comb through dry hair. In each case, objects become “electrified” as surfaces rub against one another.
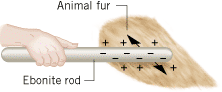 | | Figure 18.2
When an ebonite rod is rubbed against animal fur, electrons from atoms of the fur are transferred to the rod. This transfer gives the rod a negative charge (–) and leaves a positive charge (+) on the fur. |
|
When an ebonite rod is rubbed with animal fur, the rubbing process serves only to separate electrons and protons already present in the materials. No electrons or protons are created or destroyed. Whenever an electron is transferred to the rod, a proton is left behind on the fur. Since the charges on the electron and proton have identical magnitudes but opposite signs, the algebraic sum of the two charges is zero, and the transfer does not change the net charge of the fur/rod system. If each material contains an equal number of protons and electrons to begin with, the net charge of the system is zero initially and remains zero at all times during the rubbing process.
Electric charges play a role in many situations other than rubbing two surfaces together. They are involved, for instance, in chemical reactions, electric circuits, and radioactive decay. A great number of experiments have verified that in any situation, the law of conservation of electric charge is obeyed.
| LAW OF CONSERVATION OF ELECTRIC CHARGE |
|
During any process, the net electric charge of an isolated system remains constant (is conserved).
|
It is easy to demonstrate that two electrically charged objects exert a force on one another. Consider Figure 18.3a, which shows two small balls that have been oppositely charged and are light and free to move. The balls attract each other. On the other hand, balls with the same type of charge, either both positive or both negative, repel each other, as parts b and c of the drawing indicate. The behavior depicted in Figure 18.3 illustrates the following fundamental characteristic of electric charges:
 | Like charges repel and unlike charges attract each other.
|
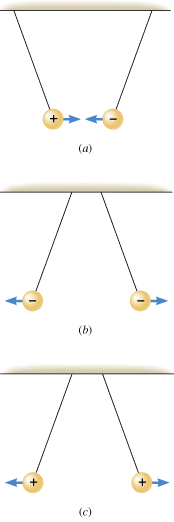 | | Figure 18.3
(a) A positive charge (+) and a negative charge (–) attract each other. (b) Two negative charges repel each other. (c) Two positive charges repel each other. |
|
 CONCEPTS AT A GLANCE Like other forces that we have encountered, the electric force (also called the electrostatic force) can alter the motion of an object. It can do so by contributing to the net external force SF that acts on the object. Newton’s second law, SF
CONCEPTS AT A GLANCE Like other forces that we have encountered, the electric force (also called the electrostatic force) can alter the motion of an object. It can do so by contributing to the net external force SF that acts on the object. Newton’s second law, SF =
= ma, specifies the acceleration a that arises because of the net external force. The Concepts-at-a-Glance chart in Figure 18.4 is an expanded version of the charts in Figures 4.9, 10.4, and 11.5, and emphasizes that any external electric force that acts on an object must be included when determining the net external force to be used in the second law.
ma, specifies the acceleration a that arises because of the net external force. The Concepts-at-a-Glance chart in Figure 18.4 is an expanded version of the charts in Figures 4.9, 10.4, and 11.5, and emphasizes that any external electric force that acts on an object must be included when determining the net external force to be used in the second law.
|
| Figure 18.4
CONCEPTS AT A GLANCE External electrostatic forces that act on an object must be included in the net external force when using Newton’s second law to determine acceleration. In obtaining a DNA “fingerprint” (see photograph) using electrophoresis, an electrostatic force accelerates electrically charged DNA fragments to different terminal speeds in an agarose gel. (© Geoff Tompkinson/Photo Researchers) |
|
A new technology based on the electric force may revolutionize the way books and other printed matter are made. This technology, called electronic ink, allows letters and graphics on a page to be changed instantly, much like the symbols displayed on a computer monitor. Figure 18.5a illustrates the essential features of electronic ink. It consists of millions of clear microcapsules, each having the diameter of a human hair and filled with a dark inky liquid. Inside each microcapsule are several dozen extremely tiny white beads that carry a slightly negative charge. The microcapsules are sandwiched between two sheets, an opaque base layer and a transparent top layer, at which the reader looks. When a positive charge is applied to a small region of the base layer, as shown in part b of the drawing, the negatively charged white beads are drawn to it, leaving dark ink at the top layer. Thus, a viewer sees only the dark liquid. When a negative charge is applied to a region of the base layer, the negatively charged white beads are repelled from it and are forced to the top of the microcapsules; now a viewer sees a white area due to the beads. Thus, electronic ink is based on the principle that like charges repel and unlike charges attract each other; a positive charge causes one color to appear, and a negative charge causes another color to appear. Each small region, whether dark or light, is known as a pixel (short for “picture element”). Computer chips provide the instructions to produce the negative and positive charges on the base layer of each pixel. Letters and graphics are produced by the patterns generated with the two colors.
|
| Figure 18.5
(a) Electronic ink consists of microcapsules filled with a dark, inky liquid and dozens of white beads. (b) Dark and light pixels are formed when positive and negative charges are placed in the base layer by electronic circuitry. |
|
 |
| Copyright © 2000-2003 by John Wiley & Sons, Inc. or related companies. All rights reserved. |



![]() CONCEPTS AT A GLANCE Like other forces that we have encountered, the electric force (also called the electrostatic force) can alter the motion of an object. It can do so by contributing to the net external force SF that acts on the object. Newton’s second law, SF
CONCEPTS AT A GLANCE Like other forces that we have encountered, the electric force (also called the electrostatic force) can alter the motion of an object. It can do so by contributing to the net external force SF that acts on the object. Newton’s second law, SF![]() =
=![]() ma, specifies the acceleration a that arises because of the net external force. The Concepts-at-a-Glance chart in Figure 18.4 is an expanded version of the charts in Figures 4.9, 10.4, and 11.5, and emphasizes that any external electric force that acts on an object must be included when determining the net external force to be used in the second law.
ma, specifies the acceleration a that arises because of the net external force. The Concepts-at-a-Glance chart in Figure 18.4 is an expanded version of the charts in Figures 4.9, 10.4, and 11.5, and emphasizes that any external electric force that acts on an object must be included when determining the net external force to be used in the second law.