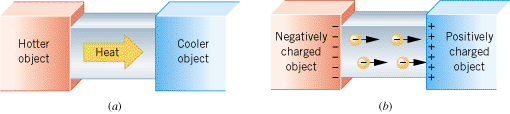
| Figure 18.6 (a) Heat is conducted from the hotter end of the metal bar to the cooler end. (b) Electrons are conducted from the negatively charged end of the metal bar to the positively charged end. |
18.3. Conductors and Insulators
Not only can electric charge exist on an object, but it can also move through an object. However, materials differ vastly in their abilities to allow electric charge to move or be conducted through them. To help illustrate such differences in conductivity, Figure 18.6a recalls the conduction of heat through a bar of material whose ends are maintained at different temperatures. As Section 13.2 discusses, metals conduct heat readily and, therefore, are known as thermal conductors. On the other hand, substances that conduct heat poorly are referred to as thermal insulators.
 |
|
A situation analogous to the conduction of heat arises when a metal bar is placed between two charged objects, as in Figure 18.6b. Electrons are conducted through the bar from the negatively charged object toward the positively charged object. Substances that readily conduct electric charge are called electrical conductors. Although there are exceptions, good thermal conductors are generally good electrical conductors. Metals such as copper, aluminum, silver, and gold are excellent electrical conductors and, therefore, are used in electrical wiring. Materials that conduct electric charge poorly are known as electrical insulators. In many cases, thermal insulators are also electrical insulators. Common electrical insulators are rubber, many plastics, and wood. Insulators, such as the rubber or plastic that coats electrical wiring, prevent electric charge from going where it is not wanted.
The difference between electrical conductors and insulators is related to atomic structure. As electrons orbit the nucleus, those in the outer orbits experience a weaker force of attraction to the nucleus than do those in the inner orbits. Consequently, the outermost electrons (also called the valence electrons) can be dislodged more easily than the inner ones. In a good conductor, some valence electrons become detached from a parent atom and wander more or less freely throughout the material, belonging to no one atom in particular. The exact number of electrons detached from each atom depends on the nature of the material, but is usually between one and three. When one end of a conducting bar is placed in contact with a negatively charged object and the other end in contact with a positively charged object, as in Figure 18.6b, the “free” electrons are able to move readily away from the negative end and toward the positive end. The ready movement of electrons is the hallmark of a good conductor. In an insulator the situation is different, for there are very few electrons free to move throughout the material. Virtually every electron remains bound to its parent atom. Without the “free” electrons, there is very little flow of charge when the material is placed between two oppositely charged bodies, so the material is an electrical insulator.