18.5. Coulomb's Law
 |
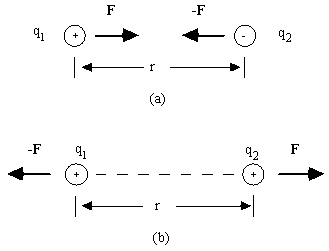
Consider two "point charges", q1 and q2, separated by a distance r, as shown in the diagram at the right. If the charges have unlike signs, as in part (a) of the diagram, each charge is attracted to the other by a force that is directed along the line between them; +F is the electric force exerted on charge 1 by charge 2 and -F is the electric force exerted on charge 2 by charge 1. If the charges have the same sign, as in (b), each charge is repelled by the other. The forces +F and -F form an action-reaction pair as predicted by Newton's third law.
The magnitude of the force (either attractive or repulsive) is given by Coulomb's law, i.e.,
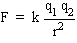 |
where k is a constant of proportionality whose value in SI units is
What is the force between charges
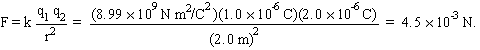 |
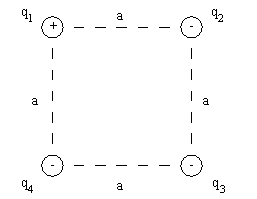 |
Consider four point charges arranged in the corners of a square, as shown in the diagram. Using,
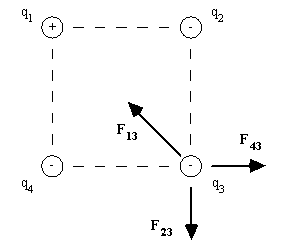 |
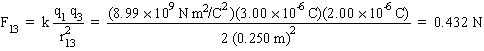 |
 |
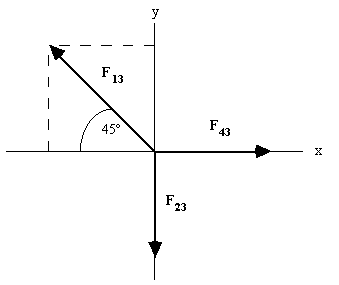 |
Force |
x component |
y component |
|
F13 |
- (0.432 N) cos 45°= - 0.305 N |
+ (0.432 N) sin 45°= + 0.305 N |
|
F23 |
= 0 |
= - 0.345 N |
|
F43 |
= + 0.345 N |
= 0 |
|
F |
= + 0.040 N |
= - 0.040 N |
|
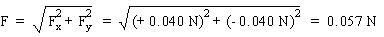 |
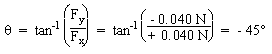 |
So the general procedure for finding the force on a point charge due to two or more other point charges is to find the vector sum of the individual forces. The magnitudes of the forces are calculated from Coulomb's law. The directions of the forces are determined from the sign of each individual charge. That is, the force is either one of attraction or repulsion, depending on whether each pair of charges has unlike or like signs, respectively.