3.10 Hydrogen Spectral Series
Pre-Lecture Reading 3.10
-
•Astronomy Today, 8th Edition (Chaisson & McMillan)
-
•Astronomy Today, 7th Edition (Chaisson & McMillan)
-
•Astronomy Today, 6th Edition (Chaisson & McMillan)
Video Lecture
-
•Hydrogen Spectral Series (14:21)
Supplementary Notes
Why Hydrogen?
-
•Hydrogen is the most abundant element in the universe, by far.
-
•Hydrogen is also the simplest element.
Lyman Series
-
•See Lyman Series.
-
•Consider transparent, hydrogen gas. If cooler than about 10,000 K, most of its electrons will be in the ground state.
-
•A continuum of light passing through this gas will consequently result in Lyman series absorption.
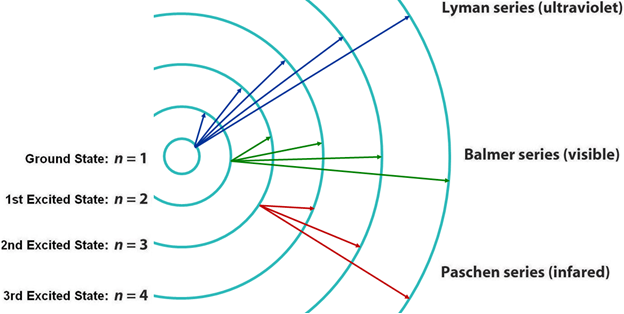
Figure 1
Lyman series transitions
-
•Lyman alpha (Lyα)
-
•Ground state ↔ 1st excited state
-
•10.2-eV or 121.6-nm photon
-
-
•Lyman beta (Lyβ)
-
•Ground state ↔ 2nd excited state
-
•12.1-eV or 102.6-nm photon
-
-
•Lyman gamma (Lyγ)
-
•Ground state ↔ 3rd excited state
-
•12.8-eV or 97.3-nm photon
-
-
•...
-
•Lyman limit
-
•Ground state ↔ ionization
-
•13.6-eV or 91.2-nm photon
-
These are all ultraviolet photons.
Balmer Series
-
•Consider hotter, transparent, hydrogen gas. If about 10,000 K, most of its electrons will be in the 1st excited state.
-
•A continuum of light passing through this gas will consequently result in Balmer series absorption.
Balmer series transitions
-
•Balmer alpha (Hα)
-
•1st excited state ↔ 2nd excited state
-
•1.9-eV or 656.5-nm photon
-
-
•Balmer beta (Hβ)
-
•1st excited state ↔ 3rd excited state
-
•2.6-eV or 486.3-nm photon
-
-
•Balmer gamma (Hγ)
-
•1st excited state ↔ 4th excited state
-
•2.9-eV or 434.2-nm photon
-
-
•...
-
•Balmer limit
-
•1st excited state ↔ ionization
-
•3.4-eV or 364.7-nm photon
-
These are almost all visible photons.
Paschen Series
-
•Consider even hotter, transparent, hydrogen gas. If about 25,000 K, most of its electrons will be in the 2nd excited state.
-
•A continuum of light passing through this gas will consequently result in Paschen series absorption.
Paschen series transitions
-
•Paschen alpha (Paα)
-
•2nd excited state ↔ 3rd excited state
-
•0.7-eV or 1875.6-nm photon
-
-
•Paschen beta (Paβ)
-
•2nd excited state ↔ 4th excited state
-
•1.0-eV or 1282.2-nm photon
-
-
•Paschen gamma (Paγ)
-
•2nd excited state ↔ 5th excited state
-
•1.1-eV or 1094.1-nm photon
-
-
•...
-
•Paschen limit
-
•2nd excited state ↔ ionization
-
•1.5-eV or 820.6-nm photon
-
These are all infrared photons.
Other Series
-
•Brackett series (3rd excited state ↔), Pfund series (4th excited state ↔), Humphreys series (5th excited state ↔), etc., are all infrared through radio photons.
-
•Beyond Humphreys, these series are increasingly difficult to measure and do not even have names.
Detection of spectral lines—in absorption or emission—yields not only composition information, but temperature information.
Exercise
Experiment with UNL's Hydrogen Atom Simulator.
Assignment 3
-
•Do Questions 7 and 8.
