3.8 Spectroscopy and Kirchhoff's Laws
Pre-Lecture Reading 3.8
-
•Astronomy Today, 8th Edition (Chaisson & McMillan)
-
•Astronomy Today, 7th Edition (Chaisson & McMillan)
-
•Astronomy Today, 6th Edition (Chaisson & McMillan)
Video Lecture
-
•Spectroscopy and Kirchhoff's Laws (17:55)
Supplementary Notes
Spectroscopy is the science of dispersing light into its component colors and measuring—and interpreting—the intensity of the light at each color (i.e., at each frequency/wavelength).
Types of Spectra
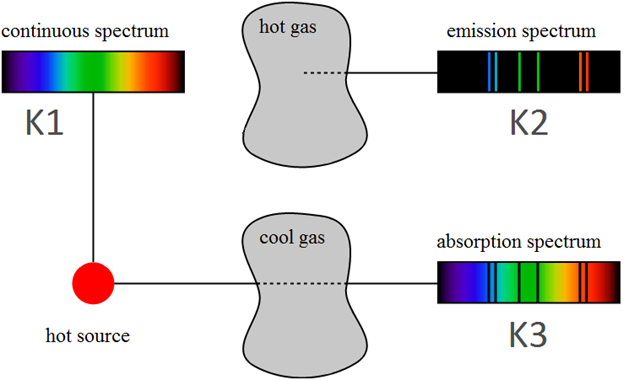
Figure 1
Kirchhoff's Laws
Kirchhoff's First Law
A continuous spectrum is produced by a:
-
•Luminous solid
-
•Luminous liquid
-
•Hot, opaque gas
-
•Hot or heated, transparent gas
-
•Backlit, cool, transparent gas
-
•Absorption of light by a cool, transparent gas heats that component of the gas, which later reemits the light in all directions.
-
•Absorption and emission lines occur at the same frequencies/wavelengths, but not necessarily in the same proportions.
Astronomical Application
-
•Absorption and emission line spectra can be used to determine both the chemical composition and the temperature of a transparent gas.
Exercise
Experiment with UNL's Three Views Spectrum Demonstrator.
Assignment 3
-
•Do Question 6.
