Appendix A – Reporting Quantitative Measurements and Results
Introduction
A numerical measurement indicates the number of units in a measurement. For example, a measurement in time could indicate the number of seconds, minutes, years, etc. Thus, units must always be reported with a measurement. The number of digits used to report the number of units indicates how precisely the measurement was made. Thus, care must be taken to report the result to the correct number of significant figures. In this appendix, we discuss how quantitative measurements should be reported.A.1 Precision
Introduction
The precision of a measurement is indicated by the number of significant figures in the reported number.Objectives
-
•Report the result of a measurement to the correct number of digits.
A.1-1. Precision
The last digit in a measurement should always be an estimate.
A.1-2. Example
Exercise A.1:
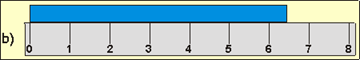
Indicate the length of the blue line to the correct precision for each measuring device.
6_1__
The blue bar is a little over 3/4 the length of the measuring device. The measuring device is 8 units long, so the blue bar is 6 or 7 units in length.
units

6.4_0.10000001__
The blue bar is slightly less than half way between 6 and 7 units, so it 6.4 or 6.5 units long.
units

6.43_0.010000001__
The end of the blue bar is about 1/3 of the way between 6.4 and 6.5, so it is 6.43 units long.
units

A.2 Significant Figures
Introduction
Not all digits in a number are necessarily significant. In this section, we see how to determine those that are.Objectives
-
•Determine the number of significant figures in a number.
A.2-1. Introduction
Significant figures are the digits that are obtained in a measurement. Thus, the precision of a measurement is indicated by the number of significant figures it contains. A measurement of 6.43 cm, which contains three significant figures, is more precise than a measurement of 6.4 cm, which contains only two significant figures. Reporting the number of significant figures in a measurement correctly is important because the number of significant figures indicates the precision of the measurement. The most common mistake made in reporting a measurement is not reporting trailing zeros to the right of the decimal. However, in a science laboratory, there is a big difference between a measurement reported to be 3 g and one reported as 3.0000 g. It is important that your number show both the magnitude and precision correctly. Consider the case where you are trying to prove or disprove a prediction that the mass of the product of a reaction should be 2.80 g. A measurement of 3.0000 g disproves the prediction, but a measurement of 3 g is inconclusive.A.2-2. Rules
There are some simple rules that allow us to determine which digits in a number are significant.-
1All nonzero numbers in a reported measurement are significant.
-
2Zeroes to the left of the decimal but to the right of all nonzero digits cannot be assumed significant. In this course, we use the practice of placing a decimal at the end of a number to indicate that the zeroes are significant. Thus, the number of significant figures in the number '300' is unclear while the number '300.' has three significant figures. The best way to indicate the number of significant figures is to use scientific notation. The numbers 3e+02 (3 × 102), 3.0e+02 (3.0 × 102), and 3.00e+02 (3.00 × 102) show a measurement of 300 to one, two and three significant figures, respectively.
-
3Leading zeroes for numbers less than one are not significant, but other zeros in the number are significant. The number 0.00012 contains only two significant figures. This becomes apparent when the number is expressed in scientific notation, 1.2e–04 (1.2 × 10–4).
-
4All zeroes to the right of the decimal of numbers greater than one are significant. The number 1.00012 contains six significant figures. If you are uncertain about the number of significant figures in a number, rewrite the number in scientific notation. All digits of a number expressed in scientific notation are significant.
A.2-3. Example
Exercise: A.2
| Number | Significant Digits |
|---|---|
| 3.000 |
4_0__
Rule 4 indicates that all zeroes to the right of the decimal are significant.
|
| 320 |
2_0__
Rule 2 indicates that zeroes to the left of the decimal but to the right of all nonzero digits cannot be assumed to be significant.
|
| 0.0005606 |
4_0__
Rule 3 indicates that leading zeroes for numbers less than 1 are not significant.
|
| 400. |
3_0__
We use a decimal at the end of a number that ends with zero to indicate that all numbers to the left of the decimal are significant.
|
A.3 Reporting Answers to Calculations
Introduction
It is frequently the case that the number to be reported is not the measurement itself, but a number obtained after a calculation involving several measurements. As with individual measurements, it is important to report the result of a calculation to the correct number of significant figures so that the reader understands the precision to which the result is known.Objectives
-
•Report the result of a calculation to the correct number of significant figures.
A.3-1. Introduction
A common mistake in reporting results of a calculation is to include all of the digits shown on the calculator. For example, consider a 5.2 mL sample that has a mass of 3.7 g. The density of the material would be determined to bed =
| 3.7 g |
| 5.2 mL |
-
1Multiplications and Divisions: The number of significant figures in the result of a calculation involving multiplication or division is equal to the number of significant figures in the least precise number used in the calculation. Thus, the density discussed in the preceding paragraph should be rounded to 0.71 g/mL because both the mass and the volume were measured to two significant figures.
-
2Additions and Subtractions: The number of decimal places in the result of an addition or subtraction is equal to the number of decimal places in the given number that has the fewest decimal places. A good way to remember this rule is to realize that the result of the addition of a significant number and an insignificant number is insignificant. If you had $3.25 and someone gave you about $2 in change, you would have a total of about $5, not $5.25, but if they gave you $2.00 in change, you would have $5.25.
A.3-2. Example
Exercise: A.3
Express each result to the correct number of significant figures.
| Operation | Calculator | Result | ||
|---|---|---|---|---|
| (2.7)(6.345) | 17.1315 |
17.1315__2_
The number of significant figures in the result of a multiplication equals the number of significant figures in the number with the least number of significant figures. 2.7 has only two significant figures.
|
||
| 1.0 – 0.0003 | 0.9997 |
0.9997___1
1.0 is significant only to a tenth, so the answer is significant to only a tenth.
|
||
| 12.3 – 11.2634 | 1.0366 |
1.0366_.0366__1
12.3 is significant to only a tenth, so the answer is significant to only a tenth.
|
||
| 8.76 + 7.13 | 15.89 |
15.89___2
Both numbers are significant to the hundredths place, so the answer is significant to the hundredths place.
|
||
| 8.5128/3.20 | 2.66025 |
2.66025__3_
3.20 has only three significant figures, so the answer can have only three significant figures.
|
||
|
3.444297e–05 |
3.444297e-05_5e-6_1_
The result of the subtraction is 0.0008, which has only one significant figure. Thus, the result of the division can have only one significant figure.
|
A.4 Rounding Errors
Introduction
When intermediate values in a calculation involving several steps must also be reported, they should be reported to the correct number of significant figures. However, use of rounded values in subsequent calculations can lead to significant rounding errors.Objectives
-
•Minimize rounding errors in consecutive calculations.
A.4-1. Example
Do not use the rounded values for intermediate results when doing sequential calculations.
Example:
A mixture contains 4.0 g of N2 (Mm = 28.0 g/mol) and 4.0 g of O2 (Mm = 32.0 g/mol).
How many moles of each gas are present in the mixture? Divide the mass by the molar mass to obtain the number of moles of each gas. The results of the calculation as shown on a calculator are: moles of N2 = 4.0/28.0 = 0.14286 mol and moles of O2 = 4.0/32.0 = 0.125 mol. However each answer is good to only two significant figures, so the number of moles of each gas would be rounded to 0.14 mol N2 and 0.13 mol O2. What is value of the ratio of moles of O2 to moles of N2 in the mixture?
A mixture contains 4.0 g of N2 (Mm = 28.0 g/mol) and 4.0 g of O2 (Mm = 32.0 g/mol).
How many moles of each gas are present in the mixture? Divide the mass by the molar mass to obtain the number of moles of each gas. The results of the calculation as shown on a calculator are: moles of N2 = 4.0/28.0 = 0.14286 mol and moles of O2 = 4.0/32.0 = 0.125 mol. However each answer is good to only two significant figures, so the number of moles of each gas would be rounded to 0.14 mol N2 and 0.13 mol O2. What is value of the ratio of moles of O2 to moles of N2 in the mixture?
Using the rounded intermediate values:
= 0.93 mol O2/mol N2
| 0.13 mol O2 |
| 0.14 mol N2 |
With no rounding of intermediate values:
= 0.88 mol O2/ mol N2
| 4.0/32.0 mol O2 |
| 7.0/28.0 mol N2 |