Chapter 9 – Electrochemistry
Introduction
Electron transfer, or redox, reactions form one of the broadest and most important classes of reactions in chemistry. All reactions involving oxygen, such as combustion and corrosion, are redox reactions. Photosynthesis, respiration, and the breakdown of food are biological processes that involve sequences of electron transfer reactions to transport and utilize energy from the sun. Electrochemistry is the marriage of electron transfer and electrical conductivity through a circuit, so all batteries are electrochemical devices. In this chapter, we review some of the topics introduced in Chapter 11 of CAMS, and extend our study of electron transfer to topics such as how concentrations affect cell potentials and how applied voltages can be used to plate one metal on another or to carry out non-spontaneous redox reactions.9.1. Redox Review
Introduction
Electrochemistry was introduced in detail in CAMS Chapter 11, and a knowledge of that material is essential to the understanding of this chapter. While a brief summary is presented in this section, a more thorough background can be acquired by viewing the videos that can be found in the Sections of CAMS that are referenced in the following prerequisites. It is strongly recommended that you view these videos for a better review.Prerequisites
-
•CAMS 11.1-1 Introduction to Electron Transfer and Redox Reactions
-
•CAMS 11.4-1 Standard Reduction Potentials
-
•CAMS 11.5-1 Reactivity and Standard Reduction Potentials
-
•CAMS 11.5-2 Writing Redox Chemical Equations
9.1-1. Definitions
-
•During a redox reaction, electrons are transferred from an electron donor to an electron acceptor.
-
•Donors lose electrons in a process known as oxidation, while the acceptors gain electrons in a process known as reduction.
-
•Reactions involving oxidation and reduction are called redox reactions.
-
•An acceptor cannot be reduced unless a donor is oxidized because electron transfer requires both a source and a destination for the transferred electrons.
-
•The reduction of an acceptor causes the oxidation of a donor, so acceptors are called oxidizing agents or oxidants.
-
•The oxidation of the donor causes the reduction of an acceptor, so donors are called reducing agents or reductants.
-
•Reductants lose electrons to become oxidants, and oxidants gain electrons to become reductants.
-
•A reducing agent and the oxidizing agent that it becomes when it is oxidized form a redox couple, which we will represent with the reduced form of the couple (RED) followed by a diagonal line then the oxidized form (OX) as in Cu/Cu2+.
9.1-2. Proton and Electron Transfer Compared
There are many similarities between Brønsted acid-base reactions and electron transfer reactions. In acid-base reactions, protons (positive charges) are transferred from a proton donor (acid) to a proton acceptor (base), while in redox reactions, electrons (negative charges) are transferred from an electron donor to an electron acceptor. In an acid-base reaction, the reacting acid and the produced base are called a conjugate acid-base pair, and in a redox reaction, the reacting donor and produced acceptor are called a redox couple. Acid-base reactions are extensive when the reacting acid and base are stronger than the produced acid and base, and redox reactions are extensive when the reacting oxidizing and reducing agents are stronger than the produced oxidizing and reducing agents.9.1-3. The Electron Transfer Process
Figure 9.1 represents the electron transfer from an isolated iron atom to an isolated copper(II) ion to produce an iron(II) ion and a copper atom. The electrons are on Fe initially, so it is the donor (reducing agent or reductant). The produced Fe2+ has empty redox orbitals, so it can function as an acceptor (oxidizing agent or oxidant). Thus, Fe is a donor, and Fe2+ is the produced acceptor, so Fe/Fe2+ is one redox couple in the reaction. The acceptor orbitals on Cu2+ are empty, but they are occupied in Cu. Thus, Cu2+ is the initial acceptor and Cu is the produced donor, so Cu/Cu2+ is the other redox couple in the reaction. The electrons are at higher energy on the donor (Fe) than on the acceptor (Cu), so the electron transfer is downhill in energy, exothermic, and spontaneous. The electron transfer from Cu to Fe2+ is uphill in energy, endothermic, and NOT spontaneous.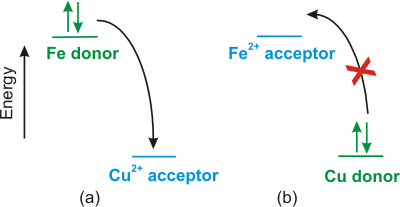
Figure 9.1: Electrons Transfer Spontaneously to Lower Energy
9.1-4. Process in Solution
We have considered electron transfer between isolated atoms and ions because the picture is far more complicated when metals and solutions are involved. Consider all that must occur when a piece of iron reacts with copper(II) ions in aqueous solution. Metallic bonds must be broken in the iron to produce an iron atom, the iron atom must lose two electrons to form Fe2+, a cavity must be created in the water to accommodate the ion, and the Fe2+ ion must enter the cavity and be hydrated. Similar processes must occur in the reverse direction when the Cu2+ ion is reduced to copper metal. Thus, the situation in aqueous redox reactions is far more complicated. If the energy difference between the donor and acceptor orbitals is not large, then these other factors can dominate the energy of the redox process. Consequently, the simple tendency of electrons to move from an orbital at high-energy into one at lower energy is not sufficient if the orbital energies are not very different. As in all processes carried out at constant temperature and pressure, it is the free energy change, ΔG, that is the driving force behind redox reactions.9.1-5. Galvanic Cell Review
When steel wool (Fe) is placed into a solution of Cu2+, the electron transfer takes place sponteneously, and the energy released simply warms the solution. However, that energy can be harnessed by separating the two redox couples and connecting them into an electrical circuit. Such circuits are called galvanic cells, and the video below shows all of the processes that occur in a galvanic cell constructed with a Cu/Cu2+ couple as the cathode and a Fe/Fe2+ couple as the anode. By definition:-
•The cathode is the compartment where reduction takes place, so it houses the Cu/Cu2+ couple.
-
•The anode is the compartment where oxidation takes place, so it houses the Fe/Fe2+ couple.
-
•Electron movement is anode → cathode.
-
•The CuSO4 solution loses its color as Cu2+ ions are consumed and the Cu electrode gets larger as Cu atoms are produced.
-
•The Fe electrode gets smaller as iron atoms are converted into Fe2+ ions that pass into solution.
-
•One K1+ ion enters the cathode for each electron that enters to maintain electrical neutrality.
-
•One Cl1– ion enters the anode for each electron that leaves to maintain electrical neutrality.
-
1charges (ions) migrate through the solution
-
2electrons pass between the solution and the circuit at the surface of the electrodes, and
-
3electrons move from the anode to the cathode in the external circuit.
Figure 9.2: Galvanic Cell
9.1-6. Cell Potential
The anode and cathode are each one-half of the redox process and are referred to as half-cells, and the cell potential is the potential difference between the two half-cells ℰcathode and ℰanode are known as the half-cell potentials. In a redox reaction, electrons must flow from the anode (donor) to the cathode (acceptor), but electrons are also negatively charged, so they also seek the more positive electical potential (charge). Thus, electrons transfer spontaneously to the cathode if it is at the higher electrical potential, but they must be forced to transfer if the cathode is at the lower electrical potential. A galvanic cell is a cell in which ℰcathode > ℰanode (ℰ > 0), so redox reactions in galvanic cells are spontaneous (ΔG < 0). The released free energy in a galvanic cell can be used to do work. Thus, we can conclude the following.
Galvanic cells convert chemical potential energy into electrical potential energy.
9.1-7. Electrolytic Cell
An electrolytic cell is a cell in which ℰcathode < ℰanode, so ℰ < 0, and ΔG > 0. Consequently, the redox electrons must be forced to move to the more negative potential with an external power supply, so we can conclude the following.
An electrolytic cell converts electrical potential energy into chemical potential energy.
9.1-8. Standard Reduction Potentials
The criterion for spontaneous electron transfer is ℰcathode > ℰanode. Therefore, tables of half-cell potentials can be used to predict spontaneity in electron transfer reactions. However, the potential of only half of a cell cannot be measured as both a donor and an acceptor are required, so absolute half-cell potentials cannot be determined. To solve this problem, chemists chose the standard hydrogen electrode or SHE as the reference half-cell. The half-cell potential of the SHE is assigned a value of exactly zero. A SHE is represented as:2 H1+(aq) + 2 e1− ⇌ H2(g) ℰ° = 0 V
9.1-9. Oxidizing Power and Reduction Potentials
The standard reduction potential of a redox couple is a measure of the electrical potential of the electron in the couple. Therefore, the standard cell potential can be determined from tabulated standard reduction potentials by rewriting Equation 9.1ℰ = ℰcathode − ℰanode
for standard conditions.
ℰ° = ℰ°cathode − ℰ°anode
ℰ°cathode = +0.34 V (the standard reduction potential of the Cu/Cu2+ couple) and ℰ°anode = –0.44 V (the standard reduction potential of the Fe/Fe2+ couple). Using Equation 9.1
ℰ = ℰcathode − ℰanode
, we determine that ℰ° = +0.34 – (–0.44) = +0.78 V. ℰ° is positive (the reaction is extensive) when ℰ°cathode is high and ℰ°anode is low or negative. Therefore, we can conclude the following.
Good oxidizing agents have high standard reduction potentials and good reducing agents have low or negative standard reduction potentials.
9.2 Balancing Redox Reactions
Introduction
Redox reactions can be difficult to balance because the loss and gain of redox electrons must also be balanced, but there are two methods that simplify the task: the half-reaction method, which is readily applied to ionic equations; and the oxidation state change method, which is useful when balancing reactions that do not involve ions. Balancing redox reactions with these methods is something chemists do occasionally, but not frequently, so these tools are not presented here. All balanced redox reactions in this chapter are constructed from tabulated half-reactions.Prerequisites
-
•CAMS 11.1 Electron Transfer or Redox Reactions (Explain the redox process and the common terms used to describe it.)
-
•CAMS 11.2 Half-Reactions (Write a balanced redox reaction given a table of reduction half-reactions.)
-
•CAMS 11.3 Galvanic Cells (Explain the workings of a galvanic cell.)
-
•CAMS 11.4 Standard Reduction Potentials (Explain what standard reduction potentials are and how they are used.)
-
•CAMS 11.5 Writing Redox Reactions (Use a table of standard reduction potentials to predict whether a redox reaction takes place when two substances are mixed.)
9.2-1. Method
Redox reactions are frequently balanced by using tabulated half-reactions like those given in the Resource titled Selected Standard Reduction Potentials at 298 K. To write the spontaneous reaction from two reduction half reactions:-
1Determine which half reaction has the less positive or more negative reduction potential and reverse it as it must serve as the oxidation half-reaction (anode).
-
2Determine the number of electrons transferred in the balanced chemical equation. This is the lowest common multiple (LCM) of the number gained at the cathode and the number lost at the anode.
-
3Multiply each half-reaction through by the integer required to make the number of electrons gained or lost in the half-reaction equal to the LCM determined in Step 2.
-
4Add the resulting half-reactions to obtain the cell reaction. Note that the same substance cannot appear on both sides of the equation, so H2O, H1+, and OH1– can often be canceled and not appear in the net equation.
-
5Determine the cell potential as ℰ°cell = ℰ°cathode – ℰ°anode.
9.2-2. Half-cell Potentials and Balancing Equations
A standard reduction potential is a measure of the electrical potential or free energy of a redox electron, so it is independent of the number of electrons or the side of the half-reaction on which they are found. We conclude the following important fact.
The standard reduction potential of a half-reaction is not changed when the direction of the half-reaction is reversed or when it is is multiplied by an integer.
9.2-3. Exercise
Exercise 9.1:

Write the balanced chemical equation for the spontaneous process that involves the following half reactions and determine the standard cell potential.
VO21+ + 2 H1+ + e1– ⇌ VO2+ + H2O ℰ° = +1.00 V
Cr2O72– + 14 H1+ + 6 e1– ⇌ 2 Cr3+ + 7 H2O ℰ° = +1.33 V
Cr2O72– + 14 H1+ + 6 e1– ⇌ 2 Cr3+ + 7 H2O ℰ° = +1.33 V
1) Identify the reaction that must be reversed and serves as the anode.
-
VO21+ + 2 H1+ + e1– ⇌ VO2+ + H2O
-
Cr2O72– + 14 H1+ + 6 e1– ⇌ 2 Cr3+ + 7 H2O The oxidation half reaction of a spontaneous cell must be the half reaction with the smaller (more negative) standard half-cell potential.

2) Determine the number of electrons that must be transferred in the reaction.
n =
6_0__0
One electron is lost in the oxidation and six are gained in the reduction. n is the LCM of 1 and 6.

Indicate any subscripted characters with an underscore (_) and any superscipted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.
3a) Determine the anode half reaction as it occurs in the overall reaction (after reversing and multiplying by the appropriate integer).
|
o_6VO^2+_s
6VO2+ + 6H2O ⇌ 6VO21+ + 12H1+ + 6e1–
|
+ |
o_6H_2O_s
6VO2+ + 6H2O ⇌ 6VO21+ + 12H1+ + 6e1–
|
→ |
o_6VO_2^1+_s
6VO2+ + 6H2O ⇌ 6VO21+ + 12H1+ + 6e1–
|
+ |
o_12H^1+_s
6VO2+ + 6H2O ⇌ 6VO21+ + 12H1+ + 6e1–
|
+ |
6_0__
6VO2+ + 6H2O ⇌ 6VO21+ + 12H1+ + 6e1–
e1–
|
3b) Determine the cathode half reaction as it occurs in the overall reaction (after multiplying by the appropriate integer).
|
o_Cr_2O_7^2-_s
Cr2O72– + 14H1+ + 6e1– ⇌ 2Cr3+ + 7H2O
|
+ |
o_14H^1+_s
Cr2O72– + 14H1+ + 6e1– ⇌ 2Cr3+ + 7H2O
|
+ |
6_0__
Cr2O72– + 14 H1+ + 6 e1– ⇌ 2 Cr3+ + 7 H2O
e1–
|
→ |
o_2Cr^3+_s
Cr2O72– + 14H1+ + 6e1– ⇌ 2Cr3+ + 7H2O
|
+ |
o_7H_2O_s
Cr2O72– + 14H1+ + 6e1– ⇌ 2Cr3+ + 7H2O
|
4) Write the chemical equation (Red1 + Ox2 + Other → Ox1 + Red2 + Other) for the spontaneous process.
|
o_6VO^2+_s
6VO2+ + Cr2O72- + 2H1+ ⇌ 6VO21+ + 2Cr3+ + H2O
|
+ |
o_Cr_2O_7^2-_s
6VO2+ + Cr2O72- + 2H1+ ⇌ 6VO21+ + 2Cr3+ + H2O
|
+ |
o_2H^1+_s
6VO2+ + Cr2O72- + 2H1+ ⇌ 6VO21+ + 2Cr3+ + H2O
|
→ |
o_6VO_2^1+_s
6VO2+ + Cr2O72- + 2H1+ ⇌ 6VO21+ + 2Cr3+ + H2O
|
+ |
o_2Cr^3+_s
6VO2+ + Cr2O72- + 2H1+ ⇌ 6VO21+ + 2Cr3+ + H2O
|
+ |
o_H_2O_s
6VO2+ + Cr2O72- + 2H1+ ⇌ 6VO21+ + 2Cr3+ + H2O
|
5) What is the standard cell potential?
ℰ° =
0.33_0__
Use Equation 9.1:ℰ° = 1.33 – 1.00
V

9.2-4. Exercise
Exercise 9.2:

Given the following half-reactions,
write the overall reaction for the oxidation of gold by permanganate ion.
Au3+ + 3 e1– ⇌ Au
MnO41– + 8 H1+ + 5 e1– ⇌ Mn2+ + 4 H2O
MnO41– + 8 H1+ + 5 e1– ⇌ Mn2+ + 4 H2O
1) The lowest common multiple of electrons gained and lost is:
n =
15_0__
the LCM of 3 and 5 is 15

2) Which half reaction must be reversed to an oxidation?
-
Au3+ + 3 e1– ⇌ Au
-
MnO41– + 8 H1+ + 5 e1– ⇌ Mn2+ + 4 H2O The problem specifies the oxidation of gold.

Indicate any subscripted characters with an underscore (_) and any superscipted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.
3) The anode half reaction as it occurs in the overall reaction (after reversing and multiplying by the appropriate integer):
|
o_5Au_s
Multiply reaction by 5 to get 15 electrons and reverse it to make it the oxidation: 5Au → 5Au3+ + 15e1–
|
→ |
o_5Au^3+_s
Multiply reaction by 5 to get 15 electrons and reverse it to make it the oxidation: 5Au → 5Au3+ + 15e1–
|
+ |
15_0__
Multiply reaction by 5 to get 15 electrons and reverse it to make it the oxidation: 5Au → 5Au3+ + 15e1–
e1–
|
4) The cathode half reaction as it occurs in the overall reaction (after multiplying by the appropriate integer):
|
o_3MnO_4^1-_s
Multiply by 3 to get 15 electrons: 3MnO41– + 24H1+ + 15e1– → 3Mn2+ + 12H2O
|
+ |
o_24H^1+_s
Multiply by 3 to get 15 electrons: 3MnO41– + 24H1+ + 15e1– → 3Mn2+ + 12H2O
|
+ |
15_0__
Multiply by 3 to get 15 electrons: 3MnO41– + 24H1+ + 15e1– → 3Mn2+ + 12H2O
e1–
|
→ |
o_3Mn^2+_s
Multiply by 3 to get 15 electrons: 3MnO41– + 24H1+ + 15e1– → 3Mn2+ + 12H2O
|
+ |
o_12H_2O_s
Multiply by 3 to get 15 electrons: 3MnO41– + 24H1+ + 15e1– → 3Mn2+ + 12H2O
|
5) Write the chemical equation (Red1 + Ox2 + Other → Ox1 + Red2 + Other) for the spontaneous process.
|
o_5Au_s
5Au + 3MnO41– + 24H1+ → 5Au3+ + 3Mn2+ + 12H2O
|
+ |
o_3MnO_4^1-_s
5Au + 3MnO41– + 24H1+ → 5Au3+ + 3Mn2+ + 12H2O
|
+ |
o_24H^1+_s
5Au + 3MnO41– + 24H1+ → 5Au3+ + 3Mn2+ + 12H2O
|
→ |
o_5Au^3+_s
5Au + 3MnO41– + 24H1+ → 5Au3+ + 3Mn2+ + 12H2O
|
+ |
o_3Mn^2+_s
5Au + 3MnO41– + 24H1+ → 5Au3+ + 3Mn2+ + 12H2O
|
+ |
o_12H_2O_s
5Au + 3MnO41– + 24H1+ → 5Au3+ + 3Mn2+ + 12H2O
|
9.3 Free Energy and Electrochemical Cells
Introduction
We now continue our treatment of galvanic cells and begin our discussion of their thermodynamics.Objectives
-
•Determine the anode and cathode reactions of the spontaneous cell given the standard reduction potentials of the two half-reactions.
-
•Express an electrochemical cell in the abbreviated form.
-
•Determine the free energy of a redox reaction from its cell potential, and vice versa.
9.3-1. Electrodes
Electron transfer reactions in electrochemical cells are known as electrochemical reactions. Electrochemical reactions occur at the surfaces of the two electrodes in the cell as electrons are injected into the external circuit at the anode as the donor is oxidized and withdrawn from the circuit at the cathode as the acceptor is reduced. Electrodes that take part in the electrochemical reactions are said to be active electrodes. For example, the iron and copper electrodes in the galvanic cell discussed in the previous section are active electrodes because both are involved in the reaction. However, there are many instances where neither member of a redox couple is a conducting solid, but an electrode is still required to serve as the interface between the reaction and the circuit. In these cases, an inert metal is normally used as a passive electrode. Passive electrodes do not participate in the reaction; they simply serve as a conducting surface through which electrons are injected into either the circuit or the reaction. For example, a Pt electrode is commonly used in a Standard Hydrogen Electrode (SHE) because there is no metal surface in the half-reaction (2 H1+ + 2 e1– ⇌ H2).9.3-2. Electrochemical Cell Abbreviation
Electrochemical cells are quite common and are often represented in the following abbreviated form:|
|
A single vertical line represents a phase change (solid/solution), and a double vertical line represents a liquid junction (salt bridge). The order in which components are listed is easily remembered if you remember your ABC's (Anode | Bridge | Cathode). As an example, the cell shown in Figure 9.2 is represented as
Fe | Fe2+(1 M) || Cu2+(1 M) | Cu
Pt | H2(1 atm), H1+(1 M) || Cu2+(1 M) | Cu
9.3-3. Cell Abbreviation Exercise
Exercise 9.3:
Write the chemical equation for the following redox process:
Lowest common multiple for electrons gained and lost.
Al | Al3+(1 M) || Sn2+(1 M) | Sn
n =
6_0__
LCM of 2 and 3

Write the chemical equation (Red1 + Ox2 → Ox1 + Red2). (Indicate any subscripted characters with an underscore (_) and any superscipted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.)
|
o_2Al_s
2Al + 3Sn2+ → 2Al3+ + 3Sn
|
+ |
o_3Sn^2+_s
2Al + 3Sn2+ → 2Al3+ + 3Sn
|
→ |
o_2Al^3+_s
2Al + 3Sn2+ → 2Al3+ + 3Sn
|
+ |
o_3Sn_s
2Al + 3Sn2+ → 2Al3+ + 3Sn
|
The standard reduction potentials of Al and Sn are –1.66 V and –0.14 V, respectively. What is the cell potential?
ℰ° =
1.52_0__
Use Equation 9.1. ℰcathode = –0.14 V and ℰanode = –1.66 V.
V

9.3-4. Free Energy
Recall from Equation 4.9 that the maximum amount of work that can be obtained from a process is the amount of free energy released during the process. Thus, the amount of work that can be done by the transferred electrons in a spontaneous redox reaction is: Historically, the work done by the transferred electrons was expressed in terms of charge and electrical potential difference, ℰ. The absolute value of the charge of one mole of electrons is called the faraday and given the symbol ℱ. The faraday is Avogadro's number times the absolute value of the charge on a single electron:ℱ = 6.02214 × 1023 electrons/mol × 1.60218 × 10−19 C/electron = 9.6485 × 104 C/mol
9.3-5. Free Energy from Cell Potential Exercise
Exercise 9.4:
ΔG° =
-880_0_3_
Use Equation 9.4, n = 6, and convert to kJ.ΔG° = –(6 mol e1–)(96,500 C/mol e1–)(1.52 J/C) = –8.80 e05 J = –880. kJ
kJ

9.4 The Effect of Concentration on Cell Potential
Introduction
The cell potential is a measure of the chemical free energy of a redox reaction, and we now apply the relationships between the reaction quotient, the equilibrium constant, and the free energy of reaction presented in Chapter 4 to electrochemical processes. These relationships allow us to derive an expression for the effect of concentration on cell potential.Objectives
-
•Determine the cell potential of a redox reaction given the standard cell potential and the concentrations of all reactants and products.
9.4-1. Nernst Equation
To determine the effect of concentrations on cell potentials, we start with Equation 4.13:ΔG = ΔG° + RT ln Q
−nℱℰ = −nℱℰ° + RT ln Q
ℰ = ℰ° −
ln Q
| RT |
| nℱ |
9.4-2. Determine Potential After Cell Discharge Exercise
Exercise 9.5:
Consider the following cell:
What is the cell potential if the cell is discharged from standard conditions to the point where [Sn2+] = 0.01 M? Assume that the volumes of the two half-cells are the same when determining Δ.
Al | Al3+ (1.00 M) || Sn2+ (1.00 M) | Sn ℰ° = 1.52 V
First, fill in the reaction table to determine the final concentration of Al3+.
| 2 Al | + | 3 Sn2+ | → | 2 Al3+ | + | 3 Sn | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| initial |
1.00_0_3_
The cell is at standard conditions initially, so all concentrations are 1.00 M.
|
1.00_0_3_
The cell is at standard conditions initially, so all concentrations are 1.00 M.
|
M | |||||||
| Δ |
-0.99_0__
The initial and final concentrations are given as 1.00 M and 0.01 M, respectively. Δ = final – initial = 0.01 – 1.00 = –0.99 M.
|
+0.66_0__
The Δ line entry for Sn2+ is known, so this entry can be determined by stoichiometry.
0.99 mol Sn2+ react
|
M | |||||||
| final |
0.01_0__
This value is given.
|
1.66_0__
1.00 M initially + 0.66 M form = 1.66 M
|
M |
The value of Q =
3e6_0__

Q =
=
= 3e6
| [Al3+]2 |
| [Sn2+]3 |
| (1.66)2 |
| (0.01)3 |
ℰ =
1.46_0__
Substitute into Equation 9.5. 
ℰ = 1.52 −
log(3e6) = 1.46 V
V
| 0.0592 |
| 6 |
9.4-3. Cell Thermodynamics Exercise
Exercise 9.6:
Write the chemical equation for the cell reaction (Red1 + Ox2 → Ox1 + Red2). (Indicate any subscripted characters with an underscore (_) and any superscipted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.)
|
o_Pb_s
Pb + 2Cu1+ → Pb2+ + 2Cu
|
+ |
o_2Cu^1+_s
Pb + 2Cu1+ → Pb2+ + 2Cu
|
→ |
o_Pb^2+_s
Pb + 2Cu1+ → Pb2+ + 2Cu
|
+ |
o_2Cu_s
Pb + 2Cu1+ → Pb2+ + 2Cu
|
n =
2_0__
Pb → Pb2+ involves the loss of how many electrons.
electrons transferred

ΔG =
-110_0_2_
ΔG = –nℱℰ = –(2 mol)(96,500 C/mol)(0.58 J/C)(1 kJ/1000 J)
kJ

Q =
300_0_2_

Q =
=
= 3.0e2
| [Pb2+] |
| [Cu1+]2 |
| 0.12 |
| (0.020)2 |
ℰ° =
0.65_0__
Solve Equation 9.5 for ℰ° and
substitute the known values 
ℰ° = ℰ +
log Q = 0.58 +
log(300)
V
| 0.0592 |
| n |
| 0.0592 |
| 2 |
ΔG° =
-130_0_2_
Use Equation 9.4: ΔG° = –(2 mol)(96,500 C/mol)(0.65 J/C)(1 kJ/1000 J) = –125 kJ However, the answer is good to only two significant figures, -130 kJ.
kJ

Given that the standard reduction potential of Pb2+ is –0.13 V, determine the standard reduction potential of Cu1+.
ℰ° =
0.52_0__
The Cu1+/Cu couple is in the cathode, so solve Equation 9.1 for ℰ°cathode = ℰ° + ℰ°anodeSubstitute the known values: ℰ°cathode = 0.65 + (–0.13) = 0.52 V.
V

9.4-4. pH from Cell Potential Exercise
Exercise 9.7:
A pH meter is an electrochemical cell whose voltage is pH dependent. This example shows how a pH meter could be constructed from a SHE and an Ag/AgCl couple. Of course, the SHE is far too cumbersome to be used in a real pH meter, but the principle is the same.
Determine the pH of the solution in the anode compartment of the following cell if the cell potential is 0.53 V.
Pt | H2 (1.00 atm), H1+ (x M) || Cl1– (1.00 M), AgCl | Ag
Determine the half-reactions, their standard reduction potentials, the chemical equation for the overall reaction (Red1 + Ox2 → Ox1 + Red2 + Other), and its standard cell potential. Write the half-reactions so that the electrons cancel in the sum. (Selected Standard Reduction Potentials at 298 K. Indicate any subscripted characters with an underscore (_) and any superscipted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.)
| Reaction | ℰ° | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anode |
o_H_2_s
H2/H1+ couple is in the anode, so it must be written as the oxidation. Pt is a passive electrode. Anode: H2 → 2 H1+ + 2 e1–
|
→ |
o_2H^1+_s
H2/H1+ couple is in the anode, so it must be written as the oxidation. Pt is a passive electrode. Anode: H2 → 2 H1+ + 2 e1–
|
+ |
2_0__
H2/H1+ couple is in the anode, so it must be written as the oxidation. Pt is a passive electrode. Anode: H2 → 2 H1+ + 2 e1–
e1–
|
0___
The tabulated value is 0.00 V.
V
|
||||
| Cathode |
o_2AgCl_s
Ag/AgCl is in the cathode, so it must be written as the reduction. Ag is an active electrode. This is a two-electron transfer, so cathode: 2AgCl + 2e1– → 2Ag + 2Cl1–
|
+ |
2_0__
Ag/AgCl is in the cathode, so it must be written as the reduction. Ag is an active electrode. This is a two-electron transfer, so cathode: 2AgCl + 2e1– → 2Ag + 2Cl1–
e1–
|
→ |
o_2Ag_s
Ag/AgCl is in the cathode, so it must be written as the reduction. Ag is an active electrode. This is a two-electron transfer, so cathode: 2AgCl + 2e1– → 2Ag + 2Cl1–
|
+ |
o_2Cl^1-_s
Ag/AgCl is in the cathode, so it must be written as the reduction. Ag is an active electrode. This is a two-electron transfer, so cathode: 2AgCl + 2e1– → 2Ag + 2Cl1–
|
0.22_0__
The tabulated value is +0.22 V.
V
|
||
| Cell |
o_H_2_s
Sum the two half-reactions to obtain: H2 + 2AgCl → 2H1+ + 2Ag + 2Cl1–
|
+ |
o_2AgCl_s
Sum the two half-reactions to obtain: H2 + 2AgCl → 2H1+ + 2Ag + 2Cl1–
|
→ |
o_2H^1+_s
Sum the two half-reactions to obtain: H2 + 2AgCl → 2H1+ + 2Ag + 2Cl1–
|
+ |
o_2Ag_s
Sum the two half-reactions to obtain: H2 + 2AgCl → 2H1+ + 2Ag + 2Cl1–
|
+ |
o_2Cl^1-_s
Sum the two half-reactions to obtain: H2 + 2AgCl → 2H1+ + 2Ag + 2Cl1–
|
0.22_0__
Use Equation 9.1 and ℰ°cathode = +0.22 V.
V
|
n =
2_0__
H2 → 2 H1+ requires the loss of how many electrons?
electrons transferred

log Q =
-11_0_2_

log Q =
(ℰ° − ℰ) =
(0.22 − 0.53) = −10.5 = −11
| n |
| 0.0592 |
| 2 |
| 0.0592 |
pH =
5.2_0_2_
log [H1+]2 = 2 log [H1+] = –2 pH,
pH = –(log Q)/2 = –(–10.5)/2 = 5.2 (Note: log [H1+]2 = –2 pH)
log Q = log
= log
= log [H1+]2
 |
| [Cl1]2[H1+]2 |
| PH2 |
 |
 |
| (1.0)2[H1+]2 |
| 1.0 |
 |
log [H1+]2 = 2 log [H1+] = –2 pH,
pH = –(log Q)/2 = –(–10.5)/2 = 5.2 (Note: log [H1+]2 = –2 pH)
9.4-5. Qualitative Change in Cell Potential
Equation 9.5ℰ = ℰ° −
log Q
indicates that ℰ increases as log Q decreases, so any change that decreases Q (decreasing a product concentration or increasing a reactant concentration) increases the cell potential. Similarly, any change that increases Q (increasing a product concentration or decreasing a reactant concentration) decreases the cell potential.
This is the same direction that LeChâtelier's principle predicts for a product.
| 0.0592 |
| n |
Cell potential is a product of a spontaneous redox reaction, so any change in the reaction that causes the equilibrium amount of product to increase will increase the cell potential, and any change that causes the amount of product to decrease will reduce the cell potential.
9.4-6. Effect of Changing Conditions on Cell Potential Exercise
Exercise 9.8:




Indicate the effect of each change on the cell potential of the following electrochemical cell.
The balanced chemical equation (Red1 + Ox2 → Ox1 + Red2) for the cell reaction is the following. (Indicate any subscripted characters with an underscore (_) and any superscipted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.)
|
o_H_2_s
H2 + Cu2+ → 2H1+ + Cu
|
+ |
o_Cu^2+_s
H2 + Cu2+ → 2H1+ + Cu
|
→ |
o_2H^1+_s
H2 + Cu2+ → 2H1+ + Cu
|
+ |
o_Cu_s
H2 + Cu2+ → 2H1+ + Cu
|
Note that ammonia is a good Lewis base, while Cu2+ and H1+ are both Lewis acids.
-
increasing the partial pressure of H2
-
increase
-
decrease H2 is a reactant.
-
no change H2 is a reactant.

-
adding NH3 to the cathode
-
increase Cu2+ + 4NH3 → Cu(NH3)42+, which reduces [Cu2+].
-
decrease
-
no change Cu2+ + 4NH3 → Cu(NH3)42+, which reduces [Cu2+].

-
adding NH3 to the anode
-
increase
-
decrease H1+ + NH3 → NH41+, which reduces [H1+].
-
no change H1+ + NH3 → NH41+, which reduces [H1+].

-
enlarging the copper electrode
-
increase Cu does not appear in the reaction quotient.
-
decrease Cu does not appear in the reaction quotient.
-
no change

9.4-7. Changing Conditions Exercise
Exercise 9.9:





Predict the effect on the cell potential of the following cell.
The balanced chemical equation (Red1 + Ox2 → Ox1 + Red2) for the cell reaction is the following. (Indicate any subscripted characters with an underscore (_) and any superscipted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.)
|
o_Pb_s
Pb + 2Cu1+ → Pb2+ + 2Cu
|
+ |
o_2Cu^1+_s
Pb + 2Cu1+ → Pb2+ + 2Cu
|
→ |
o_Pb^2+_s
Pb + 2Cu1+ → Pb2+ + 2Cu
|
+ |
o_2Cu_s
Pb + 2Cu1+ → Pb2+ + 2Cu
|
-
enlarging the lead electrode
-
increase Lead does not appear in the reaction quotient.
-
decrease Lead does not appear in the reaction quotient.
-
no change

-
adding KCl to the anode to precipitate PbCl2
-
increase
-
decrease Precipitation reduces [Pb2+].
-
no change Precipitation reduces [Pb2+].

-
evaporating some water from the cathode
-
increase
-
decrease Evaporating water increases [Cu1+].
-
no change Evaporating water increases [Cu1+].

-
adding Pb(NO3)2 to the anode
-
increase Increases [Pb2+].
-
decrease
-
no change Increases [Pb2+].

-
adding copper wire to the cathode
-
increase Cu does not appear in the reaction quotient.
-
decrease Cu does not appear in the reaction quotient.
-
no change

9.4-8. Equilibrium Constants and Standard Cell Potentials
At equilibrium, Q = K and ΔG = 0. However, ΔG = –nℱℰ, so ℰ = 0 as well. Thus, batteries die when the reaction reaches equilibrium. Applying these equilibrium conditions to Equation 9.5ℰ = ℰ° −
log Q
, we obtain the following:
| 0.0592 |
| n |
0 = ℰ° −
log K
| 0.0592 |
| n |
9.4-9. Determining K from Standard Cell Potential Exercise
Exercise 9.10:
What is the equilibrium constant for the following reaction?
n =
6_0__
n = 6

log K =
154_0__
Use Equation 9.7. log K = 16.9nℰ° = (16.9)(6)(1.52) = 154

K =
1e154___
K = 10log K = 1 e154 (1x10154)

9.5 The Electrolytic Cell
Introduction
A second technological side of electrochemistry is based on the use of external electrical circuits to control the redox chemistry itself, including the ability to force electrons uphill in energy. The process of forcing electrons uphill in energy is called electrolysis. An electrolysis reaction is one that converts electrical potential energy into chemical potential energy. Recharging batteries, electroplating, recovering elemental metals from ores, and making chemicals are some examples of electrolysis. The electrochemical cell that is used to do an electrolysis experiment is called an electrolytic cell.Prerequisites
-
•CAMS 11.8 Electrolytic Cells (Distinguish between electrolytic and galvanic cells.)
-
•CAMS 8.6 Fermi Level (Define a metallic band and the Fermi level.)
Objectives
-
•Describe how an electrolytic cell works.
-
•Determine the minimum voltage that must be applied in an electrolytic cell.
9.5-1. Electrodes
The voltage across two electrodes measures the energy difference in their Fermi levels.
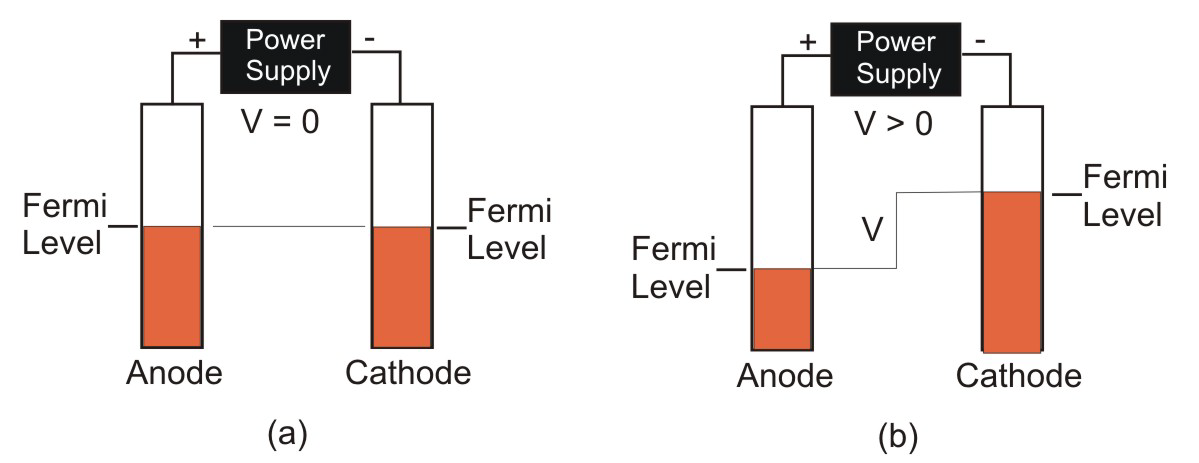
Figure 9.3: Applied Potential and Electrode Fermi Levels
The orange rectangles represent the conduction band occupancies of two pieces of the same metal. (a) When no potential is applied to the metals, their band populations and therefore their Fermi levels are the same; i.e., there is no potential difference. (b) Application of an electrical potential causes the Fermi level of the anode to drop as electrons are removed and the Fermi level of the cathode to increase as electrons are added. The potential difference in the Fermi levels equals the applied potential
9.5-2. Ion Migration
Anions migrate toward electrodes at positive potential (anode), while cations migrate toward the electrode at negative potential (cathode).
Figure 9.4: Ions move toward electrodes Click 'Apply Voltage' to see the effect of an applied voltage on the ions between the electrodes.
9.5-3. Potential Required for the Electrolysis of NaCl
When the cell potential is great enough, electrons can be forced from the cathode to the surrounding cations and to the anode from the surrounding anions. The resulting electron transfer reactions combine to produce a process called electrolysis. In an electrolysis experiment, which is carried out in an electrolytic cell, electrical potential is converted into chemical potential because the produced species are at a greater free energy than the reacting ones. We examine their function by studying the electrolysis of NaCl.2 NaCl → 2 Na + Cl2 ΔG°298 = +768 kJ
ΔG = −nℱℰ
as follows.
ℰ° =
=
= −4 V
| ΔG°f |
| nℱ |
| 768,000 J |
| (2 mol)(96,500 C/mol) |
| cathode | 2 Na1+ + 2 e1– → 2Na | ℰ°cathode = –2.7 V |
|---|---|---|
| anode | 2 Cl1– → Cl2 + 2 e1– | ℰ°anode = +1.4 V |
| cell | 2 NaCl → 2 Na1+ + 2 Cl1– | ℰ°cell = –2.7 – 1.4 = –4 V |
Both methods assume 298 K, and the half reactions represent aqueous solution. Our discussions are concerned with the molten salt, which must be heated to about 800 °C, so the applied voltage may differ from the 4 V calculated here. However, we will use 4 V for the discussion in the next section. Our conclusion is that NaCl can be electrolyzed to Na and Cl2 by the application of over 4 V from an external power supply.
9.5-4. Electrolysis of NaCl
When the applied voltage is such that the energy of the electrons at the Fermi level of the cathode is higher than the empty valence orbitals on the oxidant (Na1+), and the energy of unfilled orbitals at the Fermi level in the anode is less than the valence electrons on the reductant (Cl1–), electrons flow through the circuit, and electricity is conducted through the melt. Conduction in an electrolytic cell is called electrolytic conduction. Note that the direction of electron flow is from positive to negative as the power supply forces the electrons to lower (more negative) potential. Forcing electrons to move in this direction requires energy, which is stored in the higher free energy of the products. Thus,-
•An electrolytic cell converts the electrical potential energy generated in the power supply into chemical potential energy, which is stored in the sodium metal and chlorine gas.
Figure 9.5: Electrolysis of NaCl When the applied voltage is sufficient, electrons move from the reductant to the anode and from the cathode to the oxidant. A sodium ion (oxidant) that is reduced to sodium metal is replaced by another sodium ion in the melt as the positive cations continue to migrate toward the cathode. Similarly, a chloride ion that is oxidized to a chlorine atom at the anode is replaced by another chloride ion from the melt as the anions continue to migrate toward the anode.
9.5-5. Predicting the Required Voltage Exercise
Exercise 9.11:
What voltage must be applied to electrolyze water? The relevant half-reactions from Selected Standard Reduction Potentials at 298 K are:
2 H2O + 2 e1– ⇌ H2 + 2 OH1– ℰ° = –0.41 V
O2 + 4 H1+ + 4 e1– ⇌ 2 H2O ℰ° = +0.82 V
Electrical current is the rate at which charge flows through the circuit. It is given the symbol i and has units of amperes (A or amps). One ampere is one coulomb per second (1 A = 1 C/s). Consequently, the charge in coulombs that flows during an electrochemical experiment carried out at a fixed current of i amperes for t seconds is given by Equation 9.8.
O2 + 4 H1+ + 4 e1– ⇌ 2 H2O
Use the fact that H1+ + OH1– → H2O to write the cell reaction. (Indicate any subscripted characters with an underscore (_) and any superscipted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.)
|
o_2H_2O_s
This is the electrolysis of water, so water is the only reactant. It is also a four-electron reaction. Consequently, the O2 half-reaction must be reversed and become the anode half-reaction, and the half-reaction involving only two electrons must be doubled: 2H2O → 2H2 + O2
|
→ |
o_2H_2_s
This is the electrolysis of water, so water is the only reactant. It is also a four-electron reaction. Consequently, the O2 half-reaction must be reversed and become the anode half-reaction, and the half-reaction involving only two electrons must be doubled: 2 H2O → 2 H2 + O2
|
+ |
o_O_2_s
This is the electrolysis of water, so water is the only reactant. It is also a four-electron reaction. Consequently, the O2 half-reaction must be reversed and become the anode half-reaction, and the half-reaction involving only two electrons must be doubled: 2 H2O → 2 H2 + O2
|
Required voltage =
1.23_0__
Use Equation 9.1, ℰcathode = –0.41 V, and ℰanode = +0.82 V. ℰ = ℰcathode – ℰanode = –0.41 V – 0.82 V = –1.23 V
The minus sign simply means that the 1.23 V must be applied. V
The minus sign simply means that the 1.23 V must be applied. V
9.5-6. Overpotential
The minimum voltage required to electrolyze water was determined to be 1.23 V in the previous exercise, but the rates of the two half-reactions are negligible at that potential because the activation energies of the two half-reactions are so high. To obtain reasonable reaction rates, the applied potential must be about 1 V higher than the predicted value.-
•The amount by which the potential must be increased above the predicted value to obtain a reasonable rate of reaction is called the overpotential.
9.6 Predicting the Products of Electrolysis
Introduction
The electrolysis of a mixture of several components usually produces only one product at each electrode. In this section we discuss some guidelines to use in predicting which product forms.Objectives
-
•Predict the products of the electrolysis of a mixture.
9.6-1. Predicting the Products of Electrolysis
The predominant products formed in the electrolysis of a mixture are usually those from the components of the mixture that are most easily oxidized and reduced.-
•The species that forms at the cathode is usually the reduced form of the best oxidant (most positive or least negative reduction potential).
-
•The species that forms at the anode is usually the oxidized form of the best reductant (least positive or most negative reduction potential).
9.6-2. Predicting the Products Exercise
Exercise 9.12:






Predict the products of the electrolysis of the following.
a molten mixture of FeCl2 and NiBr2
-
•The molten mixture contains Fe2+, Ni2+, Cl1–, and Br1– ions, so the relevant half-reactions and standard reduction potentials are:
| Fe2+ + 2 e1– ⇌ Fe | ℰ° = –0.44 V |
| Ni2+ + 2 e1– ⇌ Ni | ℰ° = –0.23 V |
| Br2 + 2 e1– ⇌ 2 Br1– | ℰ° = +1.09 V |
| Cl2 + 2 e1– ⇌ 2 Cl1– | ℰ° = +1.39 V |
-
product formed at cathode
-
Fe The species formed at the cathode is the one that is the product of a reduction with the most positive standard reduction potential. Only the metals are produced by reductions.
-
Ni
-
Br2 The species formed at the cathode is the one that is the product of a reduction with the most positive standard reduction potential. Only the metals are produced by reductions.
-
Cl2 The species formed at the cathode is the one that is the product of a reduction with the most positive standard reduction potential. Only the metals are produced by reductions.

-
product formed at anode
-
Fe The species formed at the anode is the one that is the product of an oxidation with the most negative standard reduction potential. Only the halogens are produced by oxidations.
-
Ni The species formed at the anode is the one that is the product of an oxidation with the most negative standard reduction potential. Only the halogens are produced by oxidations.
-
Br2
-
Cl2 The species formed at the anode is the one that is the product of an oxidation with the most negative standard reduction potential. Only the halogens are produced by oxidations.

aqueous KI
-
•K1+ and I1– are present and water can be oxidized and reduced, so there are four possible half-reactions.
| K1+ + 1 e1– ⇌ K | ℰ° = –2.92 V |
| 2 H2O + 2 e1– ⇌ H2 + 2 OH1– | ℰ° = –0.41 V |
| I2 + 2 e1– ⇌ 2 I1– | ℰ° = +0.54 V |
| O2 + 4 H1+ + 4 e1– ⇌ 2 H2O | ℰ° = +0.88 V |
-
product formed at cathode
-
K The species formed at the cathode is the one that is the product of a reduction with the most positive standard reduction potential. The species on the right side of the half-reaction are reduced.
-
H2
-
I2 The species formed at the cathode is the one that is the product of a reduction with the most positive standard reduction potential. The species on the right side of the half-reaction are reduced.
-
O2 The species formed at the cathode is the one that is the product of a reduction with the most positive standard reduction potential. The species on the right side of the half-reaction are reduced.

-
product formed at anode
-
K The species formed at the anode is the one that is the product of an oxidation with the most negative standard reduction potential. The species on the left side of the half-reaction are oxidized.
-
H2 The species formed at the anode is the one that is the product of an oxidation with the most negative standard reduction potential. The species on the left side of the half-reaction are oxidized.
-
I2
-
O2 The species formed at the anode is the one that is the product of an oxidation with the most negative standard reduction potential. The species on the left side of the half-reaction are oxidized.

1 M CuSO4 solution
-
•The solution contains H2O, Cu2+, and SO42–, so the relevant half-reactions are:
| 2 H2O + 2 e1– ⇌ H2 + 2 OH1– | ℰ° = –0.41 V |
| Cu2+ + 2 e1– ⇌ Cu | ℰ° = +0.34 V |
| O2 + 4 H1+ + 4 e1– ⇌ 2 H2O | ℰ° = +0.88 V |
| S2O82– + 2 e1– ⇌ 2 SO42– | ℰ° = +2.01 V |
-
product formed at cathode
-
H2 The species formed at the cathode is the one that is the product of a reduction with the most positive standard reduction potential. The species on the right side of the half-reaction are reduced.
-
Cu
-
O2 The species formed at the cathode is the one that is the product of a reduction with the most positive standard reduction potential. The species on the right side of the half-reaction are reduced.
-
S2O82– The species formed at the cathode is the one that is the product of a reduction with the most positive standard reduction potential. The species on the right side of the half-reaction are reduced.

-
product formed at anode
-
H2 The species formed at the anode is the one that is the product of an oxidation with the most negative standard reduction potential. The species on the left side of the half-reaction are oxidized.
-
Cu The species formed at the anode is the one that is the product of an oxidation with the most negative standard reduction potential. The species on the left side of the half-reaction are oxidized.
-
O2
-
S2O82– The species formed at the anode is the one that is the product of an oxidation with the most negative standard reduction potential. The species on the left side of the half-reaction are oxidized.

9.7 Stoichiometry of Electrochemistry
Introduction
By the 1830s, Michael Faraday had discovered that, when a chemical reaction was harnessed to an electrical current using an electrochemical cell, the amount of electricity produced or consumed corresponded directly to the amount of chemical reactant involved. In this section, we discuss these quantitative aspects of the electrochemical cell.Objectives
-
•Determine the mass of product given the amount of charge that has flowed through the cell.
-
•Determine the number of coulombs of charge that are passed through a circuit given the current and the length of time the current flowed.
-
•Determine the mass of product given the current and the length of time the current flowed.
-
•Determine how long a given current must be applied in order to produce a given amount of product.
9.7-1. Relating the Charge Passed to the Mass formed
The electron is a reactant or a product in all half-reactions, so it is an integral part of the cell stoichiometry. However, we do not determine the mass or volume of electrons involved as we would with other reactants and products. Instead, we determine the charge in coulombs that passes during the reaction, which is measured with a coulometer. The number of coulombs that are transferred can then converted to moles of electrons with the use of the faraday, which is 96,500 C/1 mol electrons. The number of moles of electrons is converted to moles of product by using the stoichiometry of the half-reaction that involves the given species. We do the following exercises in single steps, but the answer to each step is the starting point for the following step. Thus, the steps can be combined into a single factor-label exercise. This alternate method is shown in the feedback of the final answer in each problem.9.7-2. Charge to Mass Exercise
Exercise 9.13:
What mass of copper is produced by passing 2.00 × 104 C through a solution of Cu2+?
First, convert the charge to moles of electrons:
0.207_0__

2.00e4 C ×
= 0.207 mol e1−
mol e1–
| 1 mol e1− |
| 96,500 C |
Next, use the stoichiometric factor from the appropriate half-reaction to determine the moles of copper:
0.104_0__
The half reaction is Cu2+ + 2e1– ⇌ Cu; 
0.207 mol e1− ×
= 0.104 mol Cu
mol Cu
| 1 mol Cu |
| 2 mol e1− |
Finally, use the atomic mass of copper (63.5) to convert moles of copper to grams of copper.
6.58_0__
Alternately, the entire problem could be done as a single factor label problem:
Alternately, the entire problem could be done as a single factor label problem:
2.00e4 C ×
×
×
= 6.58 g Cu
| 1 mol e1− |
| 96,500 C |
| 1 mol Cu |
| 2 mol e1− |
| 63.5 g Cu |
| mol Cu |
0.1036 mol Cu ×
= 6.58 g Cu
| 63.5 g Cu |
| mol Cu |
Alternately, the entire problem could be done as a single factor label problem:
2.00e4 C ×
×
×
= 6.58 g Cu
g Cu
| 1 mol e1− |
| 96,500 C |
| 1 mol Cu |
| 2 mol e1− |
| 63.5 g Cu |
| mol Cu |
9.7-3. Electrical Current
1 amp = 1 C/s
9.7-4. Mass of Product from Current and Time Exercise
Exercise 9.14:
What mass of gold would be plated on an earring by passing a current of 10. mA through a solution of AuCl3 for 1.5 hours?
Note: the following steps can be combined into one series of multiplications. The order can be determined using the factor label method.
charge passed =
54_0__
10. mA = 1.0e–2 C/s

1.5 hr ×
×
= 54 C
C
| 3600 s |
| 1 hr |
| 1.0e−2 C |
| 1 s |
moles of electrons passed =
5.6e-4_0__

54 C ×
= 5.6e−4 mol e1−
mol e1–
| 1 mol e1− |
| 96,500 C |
moles of gold plated =
1.9e-4_0__
Au3+ + 3e1– ⇌ Au

5.6e−4 mol e1− ×
= 1.9e−4 mol Au
mol Au
| 1 mol Au |
| 3 mol e1− |
mass of gold plated in milligrams =
37_0__
Set up as a single factor-label exercise:

1.5 hr ×
×
×
×
×
×
= 37 mg Au
atomic weight of Au = 197 | 3600 s |
| 1 hr |
| 1.0 e−2 C |
| 1 s |
| 1 mol e1− |
| 96,500 C |
| 1 mol Au |
| 3 mol e1− |
| 197 g Au |
| mol Au |
| 1000 mg |
| 1 g |
1.9e−4 mol Au ×
×
= 37 mg Au
Or, set up as a single factor-label exercise: | 197 g Au |
| mol Au |
| 1000 mg |
| 1 g |
1.5 hr ×
×
×
×
×
×
= 37 mg Au
mg Au
| 3600 s |
| 1 hr |
| 1.0 e−2 C |
| 1 s |
| 1 mol e1− |
| 96,500 C |
| 1 mol Au |
| 3 mol e1− |
| 197 g Au |
| mol Au |
| 1000 mg |
| 1 g |
9.7-5. Time Required for Electrolysis Exercise
Exercise 9.15:
How long (in minutes) should a 5.0 A current be passed through a solution of Ni(NO3)2 in order to nickel plate a bathroom fixture with 0.85 g of nickel?
Time in minutes =
9.3_0__

0.85 g Ni ×
×
×
×
×
= 9.3 min
| 1 mol Ni |
| 58.7 g Ni |
| 2 mol e1− |
| 1 mol Ni |
| 96,500 C |
| 1 mol e1− |
| 1 s |
| 5.0 C |
| 1 min |
| 60 s |
