Chapter 8 – Equilibria Containing Metal Ions
Introduction
Several different equilibria must be considered when dealing with aqueous metal ions:-
•Metal ions are Lewis acids, so they are involved in acid-base equilibria.
-
•As Lewis acids, they can react with other Lewis bases to form complex ions.
-
•Many metal ions form sparingly soluble salts, so solubility equilibria must also be considered.
8.1 Acid-Base Equilibria
Introduction
While metal ions rarely have protons to donate as required by the Brønsted acid definition, they can accept electron pairs to form covalent bonds, which makes them acidic by the Lewis definition. Thus, an aqueous solution of iron(III) nitrate is acidic even though it contains no protons.Objectives
-
•Explain the acidity of metal ions and write the acid-dissociation reaction associated with the acidity.
8.1-1. Metal Ions as Acids
Metal ions form acidic solutions because the positive charge on the metal ion weakens the O–H bonds of the bound water molecules.
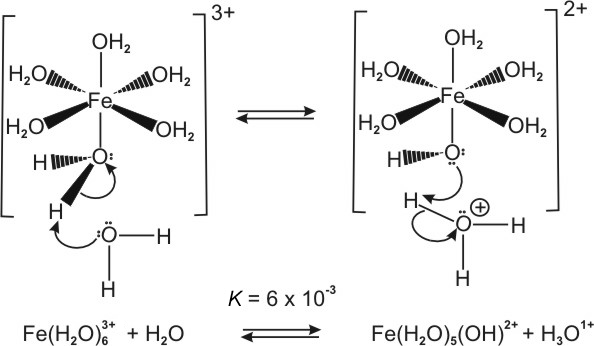
Figure 8.1: Acidity of Metal Ions
Fe(H2O)63+ + H2O ⇌ [Fe(H2O)5OH]2+ + H3O1+ K = 6e-03
| Hydrated Metal Ion | Ka |
|---|---|
| Fe(H2O)63+ | 6e–03 |
| Al(H2O)63+ | 1e–05 |
| Cu(H2O)62+ | 3e–08 |
| Zn(H2O)62+ | 1e–09 |
| Ni(H2O)62+ | 1e–10 |
Table 8.1: Acid Dissociation Constants of Hydrated Metal Ions
8.2 Dissolution and the Solubility Product Constant
Introduction
Calcium carbonate (also known as limestone) is a slightly soluble salt that is responsible for scale in containers of hard water. In addition, caves and the unusual structures in them called stalactites and stalagmites are the result of dissociation and subsequent precipitation of calcium carbonate. This important dissociation process is represented in the following equilibrium:
CaCO3(s) ⇌ Ca2+(aq) + CO32–(aq) K = [Ca2+][CO32–]
This type of process, the equilibrium between a solid and its ions in solution, is an important one and forms the topic of this section.
Objectives
-
•Write the dissociation reaction of a slightly soluble salt.
-
•Write the expression for the solubility product constant, Ksp, of a given salt.
-
•Calculate the solubility of an ionic compound given its Ksp value, and vice versa.
-
•Calculate the solubility of an ionic compound in the presence of one of its ions.
8.2-1. The Solubility Product
The equilibrium between a solid and its solution is an important one. Indeed, it is so important that the equilibrium is given a special symbol and name: the solubility product constant (Ksp). Consider the equilibrium between solid silver chloride and its solution:AgCl ⇌ Ag1+ + Cl1−.
The activity of the solid is unity, so the form of the equilibrium constant, Ksp, for the reaction is
Ksp = [Ag1+][Cl1−].
It is called the solubility product constant because it is simply a product of concentrations that indicates the solubility of the solid.
Most slightly soluble salts contain basic anions, which are involved in both acid-base equilibria with water and salt dissolution equilibria with metal ions. This can lead to a complicated set of equilibria in the case of fairly strong bases such as CO32– and PO43–. Consequently, our discussions of solubility in water centers on the solubility of neutral or only weakly basic anions, such as Cl1– and SO42–. The reaction table for the dissociation of Ag2SO4 in water has the following form:
| Ag2SO4(s) | ⇌ | 2 Ag1+ | + | SO42– | Ksp = 1.4 × 10–5 | |
|---|---|---|---|---|---|---|
| initial | enough | 0 | 0 | M |
||
| Δ | +2x |
+x |
M |
|||
| equilibrium | some | 2x |
x |
M |
Ksp = 1.4 × 10−5 = [Ag1+]2[SO42−] = (2x)2(x) = 4x3
x =
= [SO42−] =
[Ag1+]
| 3 |  |
|
| 1 |
| 2 |
-
•molar solubility of silver sulfate; x = 0.015 M
-
•[SO42–] = x = 0.015 M
-
•[Ag1+] = 2x = 0.030 M
Ksp = [Ag1+]2[SO42−] = (0.030)2(0.015) = 1.4e−05
8.2-2. Ksp from Solubility Exercise
Exercise 8.1:
What is the Ksp of MgF2 (Mm = 62.3 g/mol) if its solubility is 7.6 mg/100. mL?
The molar solubility of MgF2 =
1.2e-3_0__
7.6 mg/100. mL = 76 mg/1 L = 0.076 g/L
×
=
= 1.2e−03 M
M

| 0.076 g MgF2 |
| 1 L solution |
| 1 mol MgF2 |
| 62.3 g MgF2 |
| 1.2e−03 mol MgF2 |
| 1 L solution |
[Mg2+] =
1.2e-3_0__
There is one mole of Mg for each mole of MgF2, so
[Mg2+] = x = 1.2e–03 M. M
[Mg2+] = x = 1.2e–03 M. M
[F1–] =
2.4e-3_0__
There are two moles of F for each mole of MgF2, so
[F1–] = 2x = 2(1.2e–03) = 2.4e–03 M. M
[F1–] = 2x = 2(1.2e–03) = 2.4e–03 M. M
K =
7.3e-9_0__
Carry along extra significant figures to reduce rounding errors:
[Mg2+][F1–]2 = (1.22e–03)×(2.44e–03)2 = 7.3e–09.
[Mg2+][F1–]2 = (1.22e–03)×(2.44e–03)2 = 7.3e–09.
8.2-3. Ksp from pH Exercise
Exercise 8.2:
What is the Ksp of Fe(OH)2 if the pH of a saturated solution is 9.07? Watch out for rounding errors!
[OH1–] =
1.2e-5_0__
M

| pOH | = | 14.00 − pH |
| = | 14.00 – 9.07 | |
| = 4.93; | ||
| [OH1−] | = | 10–4.93 |
| = | 1.2e–05 M |
[Fe2+] =
5.9e-6_0__
Watch out for rounding errors! There are two moles of OH1– for every on mole of Fe2+, so [Fe2+] = [OH1–]/2 = 5.9e–06 M.
M

K =
8.1e-16_0__
Carry along extra significant figures to reduce rounding errors:
[Fe2+][OH1–]2 = (5.874e–06)×(1.175e–05)2 = 8.1e–16
[Fe2+][OH1–]2 = (5.874e–06)×(1.175e–05)2 = 8.1e–16
8.2-4. Solubility from Ksp Exercise
Exercise 8.3:
The Ksp of AgI is 8.3e–17. What is its molar solubility?
Molar solubility =
9.1e-9_0__
Ksp = [Ag1+][I1–] = x2, so x = Ksp1/2
M

8.2-5. Ksp and Strong Bases
Let us now determine the solubility of the salt of Ag3PO4; Ksp = 2.6 × 10–18. The reaction table for the dissolution has the following form:| Ag3PO4(s) | ⇌ | 3 Ag1+ | + | PO43– | |
|---|---|---|---|---|---|
| initial | enough | 0 | 0 | ||
| Δ | +3x |
+x |
|||
| equilibrium | some | 3x |
x |
Ksp = [Ag1+]3[PO43–] = (3x)3(x) = 27x4
Ksp = [Ag1+]3[PO43−]
is still valid, but the loss of PO43– in the reaction with water means that the equality 3[Ag1+] = [PO43–]
is no longer valid. This is why we restrict our discussion of solubility to neutral or weakly basic salts only. We treat the solubility of Ag3PO4 in more detail in Section 8.4.
8.2-6. Cation:Anion Ratios in Ksp
The preceding examples demonstrate that the form of the Ksp expression in terms of the molar solubility of the salt (x) depends only upon the cation:anion ratio in the salt. The Ksp expressions for the common ratios are given below.| Cation:Anion Ratio | Ksp |
|---|---|
| 1:1 | x2 |
| 1:2 or 2:1 | 4x3 |
| 1:3 or 3:1 | 27x4 |
| 2:3 or 3:2 | 108x5 |
Table 8.2: Ksp expressions in terms of molar solubilities (x) for salts with common cation:anion ratios
The relative solubilities of salts with the same cation:anion ratios can be obtained by simply looking at the magnitudes of their Ksp's. However, when the ratios are different, you must determine the value of x.
8.2-7. Determining Relative Solubilities Exercise
Exercise 8.4:



List the following salts in order of increasing solubility.
| PbCl2 | Ksp = 1.7e–5 |
| AgCl | Ksp = 1.2e–10 |
| TlCl | Ksp = 1.9e–4 |
-
Least soluble salt:
-
PbCl2
AgCl: x = (1.2e−10)1/2 ~ 1e−5 M -
AgCl
-
TlCl
AgCl: x = (1.2e−10)1/2 ~ 1e−5 M

-
Intermediate salt:
-
PbCl2
TlCl: x = (1.9e−4)1/2 = 0.014 M -
AgCl
TlCl: x = (1.9e−4)1/2 = 0.014 M -
TlCl

-
Most soluble salt:
-
PbCl2
-
AgCl
PbCl2: x = (1.7e−5)1/3 = 0.016 M -
TlCl
PbCl2: x = (1.7e−5)1/3 = 0.016 M

8.2-8. Predicting the More Soluble Salt Exercise
Exercise 8.5:




Indicate the more soluble compound in each pair.
-
PbSO4 (Ksp = 2e–8) The ion ratios are the same, so the more soluble compound is the one with the higher Ksp.
-
CaSO4 (Ksp = 2e–5)

-
BaF2 (Ksp = 2e–6)
-
PbF2 (Ksp = 4e–8) The ion ratios are the same, so the more soluble compound is the one with the higher Ksp.

-
BaCrO4 (Ksp = 2e–10) The ion ratios are NOT the same, so the solubilities must be determined. BaCrO4 isx2and Ag2CrO4 is4x3.
-
Ag2CrO4 (Ksp = 3e–12)

-
Ag3PO4 (Ksp = 2e–18)
-
Mg3(PO4)2 (Ksp = 5e–24) The ion ratios are NOT the same, so the solubilities must be determined. Ag3PO4 is27x4and Mg3(PO4)2 is108x5.

8.2-9. Common-Ion Effect
Consider the dissolution of Ag2SO4(s) in 1.0 M Na2SO4.| Ag2SO4(s) | ⇌ | 2 Ag1+ | + | SO42– | Ksp = 1.4 × 10–5 | |
|---|---|---|---|---|---|---|
| initial | enough | 0 | 1.0 | M | ||
| Δ | +2x |
+x |
M |
|||
| equilibrium | some | 2x |
0.10 + x |
M |
Ksp = [Ag1+]2[SO42–] = (2x)2(1.0 + x)
x < 0.015 M.
If we assume that x is negligible in the addition (1.0 + x ~ 1.0),
we can simplify the expression
Ksp = (2x)2(1.0)
x =
=
= 1.9 × 10−3 M
 |
|
 |
|
8.2-10. Common-Ion Solubility from pH Exercise
Exercise 8.6:
Determine the solubility of Sn(OH)2 in solutions buffered at the following pHs.
Ksp =
pH = 9.00
The concentration of the common ion:
[OH1–] =
1.0e-5_0_2_
pOH = 14.00 – 9.00 = 5.00; [OH1–] = 10–pOH = 1.0e–5
M

solubility
1.4e-18_0__

[Sn2+] =
=
= 1.4e−18 M
M
| Ksp |
| [OH1−]2 |
| 1.4e−28 |
| (1.0e−05)2 |
pH = 2.00
The concentration of the common ion:
[OH1–] =
1.0e-12_0_2_
pOH = 14.00 - 2.00 = 12.00; [OH1–] = 1.0e–12
M

solubility
1.4e-4_0__

[Sn2+] =
=
= 1.4e−04 M
M
| Ksp |
| [OH1−]2 |
| 1.4e−28 |
| (1.0e−12)2 |
8.2-11. Solubility of AgCl Exercise
Exercise 8.7:
Ksp = 1.8e–10 for AgCl. Determine the solubility of AgCl in the following:
water
solubility =
1.3e-5_0__
M

| a 1:1 salt, so x | = | Ksp1/2 |
| = | (1.8e–10)1.2 | |
| = | 1.3e–05 M |
0.10 M NaCl
solubility =
1.8e-9_0__
[Cl1–] = 0.10 M, so 
[Ag1+] =
=
= 1.8e−09 M
M
| Ksp |
| [Cl1−] |
| 1.8e−10 |
| 0.10 |
8.3 Precipitation and Separation of Ions
Introduction
Our discussions thus far have focused on the amount of a slightly soluble salt that dissolves, but now we consider the reverse process; i.e., does a precipitate form under a given set of concentrations? We do this by considering the dissociation reaction from the reverse direction; that is, we examine the formation (precipitation) of the salt.Objectives
-
•Predict whether a precipitation will occur given the concentrations of the ions and the Ksp of the salt.
-
•Determine the appropriate anion concentration to achieve maximum separation of two ions by the selective precipitation of one.
8.3-1. The Ion Product
Does a solid form under a given set of conditions, or what minimum concentration of one of the ions is required to produce a precipitate? These are two questions we will answer in this section. For example, consider a mixture that contains some solid Ag2SO4(s) suspended in a solution that is 0.01 M in Ag1+ and 0.10 M in [SO42–]. Under these conditions, does the solid dissolve or does it precipitate out of solution? To answer this question, we examine the direction in which the following reaction is proceeding under these conditions.| Ag2SO4(s) | ⇌ | 2 Ag1+ | + | SO42– | Ksp = 1.4 × 10–5 | |
|---|---|---|---|---|---|---|
| initial | ? | 0.01 | 0.10 | M |
-
•Precipitation occurs (reaction proceeds ←) if Qip > Ksp
-
•The salt dissolves (reaction proceeds →) if Qip < Ksp
8.3-2. Identifying Precipitating Hydroxides Exercise
Exercise 8.8:



An acidic solution is made 0.01 M in Cu2+, Mg2+, Fe2+, and Sn2+. The pH of the solution is then adjusted by adding NaOH. Select all hydroxides that precipitate at each of the following pHs.
pH = 4
Qip =
1e-22_0__
All hydroxides are of the form M(OH)2, so Qip =[M2+]
[OH1–]2[M2+] = 0.01 M
pOH = 14 – 4 = 10, so [OH1–] = 1e–10 M
Qip = (0.01)(1e–10)2 = 1e–22
[OH1–]2[M2+] = 0.01 M
pOH = 14 – 4 = 10, so [OH1–] = 1e–10 M
Qip = (0.01)(1e–10)2 = 1e–22
pH = 6
Qip =
1e-18_0__
All hydroxides are of the form M(OH)2, so Qip =[M2+][OH1–]2
[M2+] = 0.01 M
pOH = 14 – 6 = 8, so [OH1–] = 1e–8 M
Qip = (0.01)(1e–8)2 = 1e–18
[M2+] = 0.01 M
pOH = 14 – 6 = 8, so [OH1–] = 1e–8 M
Qip = (0.01)(1e–8)2 = 1e–18
-
only Sn(OH)2
-
Sn(OH)2 and Cu(OH)2 Only the Ksp of Sn(OH)2 is less than 1e–22.
-
Sn(OH)2, Cu(OH)2, and Fe(OH)2 Only the Ksp of Sn(OH)2 is less than 1e–22.
-
All four hydroxides precipitate. Only the Ksp of Sn(OH)2 is less than 1e–22.

-
only Sn(OH)2 The Ksp's of Sn(OH)2 and Cu(OH)2 are less than 1e–18.
-
Sn(OH)2 and Cu(OH)2
-
Sn(OH)2, Cu(OH)2, and Fe(OH)2 The Ksp's of Sn(OH)2 and Cu(OH)2 are less than 1e–18.
-
All four hydroxides precipitate. The Ksp's of Sn(OH)2 and Cu(OH)2 are less than 1e–18.

pH = 8
Qip =
1e-14_0__
All hydroxides are of the form M(OH)2, so Qip =[M2+][OH1–]2
[M2+] = 0.01 M
pOH = 14 – 8 = 6, so [OH1–] = 1e–6 M
Qip = (0.01)(1e–6)2 = 1e–14
[M2+] = 0.01 M
pOH = 14 – 8 = 6, so [OH1–] = 1e–6 M
Qip = (0.01)(1e–6)2 = 1e–14
-
only Sn(OH)2 The Ksp's of Sn(OH)2, Cu(OH)2, and Fe(OH)2 are less than 1e–14.
-
Sn(OH)2 and Cu(OH)2 The Ksp's of Sn(OH)2, Cu(OH)2, and Fe(OH)2 are less than 1e–14.
-
Sn(OH)2, Cu(OH)2, and Fe(OH)2
-
All four hydroxides precipitate. The Ksp's of Sn(OH)2, Cu(OH)2, and Fe(OH)2 are less than 1e–14.

8.3-3. Identifying Precipitating Chlorides Exercise
Exercise 8.9:

Identify any precipitates that result when 5.0 mL of 0.010 M HCl is added to 20. mL of a solution in which [Tl1+] = 0.15 M and [Pb2+] = 0.20 M.
Ksp of TlCl = 1.9e–4
Ksp of PbCl2 = 1.7e–5
Concentrations after mixing:
[Cl11–] = [Tl1+] = [Pb2+] =
[Cl11–] =
2.0e-3_0_2_
[Cl1–] = 0.05 mmol/25 mL
M

0.12_0__
[Tl1+] = 3.0 mmol in 25 mL
M

0.16_0__
[Pb2+] = 4.0 mmol in 25 mL
M

Ion-products:
TlCl:
2.4e-4_0__
(0.12)(0.0020)

PbCl2:
6.4e-7_0__
(0.16)(0.0020)2

-
The precipitate is _______________.
-
TlCl
-
PbCl2 Qip > Ksp for TlCl but not for PbCl2

8.3-4. Determining a Required Concentration
Precipitation occurs when the ion-product exceeds the solubility product, so the concentration of one reactant that is required to initiate precipitation can be determined if the concentration of the other reactant and the Ksp are known. To determine the minimum concentration required, we solve the Ksp expression for the unknown concentration, which is the maximum concentration at which no precipitation occurs. Thus, the calculation indicates both the minimum concentration required for precipitation or the maximum concentration allowed with no precipitation.8.3-5. Determining pH at which Precipitate Forms Exercise
Exercise 8.10:
At what pH does Mg(OH)2 (Ksp = 1.8 × 10–11) begin to precipitate from a solution that is 0.01 M in Mg2+?
Use the given Mg2+ concentration to determine the hydroxide ion concentration at which Qip = Ksp.
[OH1–] =
4e-5_0__

Ksp = [Mg2+][OH1−]2
[OH1−] =
=
= 4 × 10−5 M
M
 |
|
 |
|
Convert the hydroxide ion concentration into a pOH.
pOH =
4.4_0__
pOH = –log(4e–5) = 4.4

Convert the pOH into a pH.
pH =
9.6_0__
pH = 14.0 – 4.4 = 9.6
Precipitation occurs at pH values above 9.6, but not below.
Precipitation occurs at pH values above 9.6, but not below.
8.3-6. Separations
Chemists frequently need to separate a mixture into its components. In the case of aqueous mixtures of ions, this can often be accomplished by selectively precipitating the ions from the solution. We demonstrate the method by separating Sn2+ and Mg2+ ions from a solution by selectively precipitating the less soluble hydroxide by adjusting the pH to a value that results in the precipitation of only one ion. The Ksp of Sn(OH)2 is much less that that of Mg(OH)2, so we will choose to precipitate Sn(OH)2 and leave Mg2+ ions in solution. We could determine the pH at which Sn(OH)2 begins to precipitate, and adjust the pH to a slightly higher value to precipitate Sn(OH)2. However, Sn2+ ions will remain in solution with the following concentration:[Sn2+] =
| Ksp |
| [OH1−]2 |
8.3-7. Optimum Separation Exercise
Exercise 8.11:


A solution is 0.010 M each in Sn2+ and Fe2+. At what pH would optimum separation be achieved?
Ksp = 8.0e–16 for Fe(OH)2 and 1.4e–28 for Sn(OH)2.
Ksp = 8.0e–16 for Fe(OH)2 and 1.4e–28 for Sn(OH)2.
-
Which metal ion should be precipitated?
-
Sn2+
-
Fe2+ The hydroxide with the lowest Ksp will precipitate first.

-
Which of the following would be the best pH to use for the separation?
-
6 Determine the hydroxide ion concentration that would be required to precipitate the more soluble hydroxide:
[OH1−] = 
Ksp [Fe2+] = 
8 × 10−16 0.01 = 3 × 10−7 M
pOH = 6.5; pH = 7.5
Fe(OH)2 begins to precipitate at pH = 7.5. For best separation, the pH should be as high as possible to precipitate as much Sn(OH)2 as possible, but it must be belowpH = 7.5to avoid precipitating Fe(OH)2. Thus,pH = 7is the optimum pH in the selection. -
7
-
8 Determine the hydroxide ion concentration that would be required to precipitate the more soluble hydroxide:
[OH1−] = 
Ksp [Fe2+] = 
8 × 10−16 0.01 = 3 × 10−7 M
pOH = 6.5; pH = 7.5
Fe(OH)2 begins to precipitate at pH = 7.5. For best separation, the pH should be as high as possible to precipitate as much Sn(OH)2 as possible, but it must be belowpH = 7.5to avoid precipitating Fe(OH)2. Thus,pH = 7is the optimum pH in the selection. -
9 Determine the hydroxide ion concentration that would be required to precipitate the more soluble hydroxide:
[OH1−] = 
Ksp [Fe2+] = 
8 × 10−16 0.01 = 3 × 10−7 M
pOH = 6.5; pH = 7.5
Fe(OH)2 begins to precipitate at pH = 7.5. For best separation, the pH should be as high as possible to precipitate as much Sn(OH)2 as possible, but it must be belowpH = 7.5to avoid precipitating Fe(OH)2. Thus,pH = 7is the optimum pH in the selection. -
10 Determine the hydroxide ion concentration that would be required to precipitate the more soluble hydroxide:
[OH1−] = 
Ksp [Fe2+] = 
8 × 10−16 0.01 = 3 × 10−7 M
pOH = 6.5; pH = 7.5
Fe(OH)2 begins to precipitate at pH = 7.5. For best separation, the pH should be as high as possible to precipitate as much Sn(OH)2 as possible, but it must be belowpH = 7.5to avoid precipitating Fe(OH)2. Thus,pH = 7is the optimum pH in the selection. -
11 Determine the hydroxide ion concentration that would be required to precipitate the more soluble hydroxide:
[OH1−] = 
Ksp [Fe2+] = 
8 × 10−16 0.01 = 3 × 10−7 M
pOH = 6.5; pH = 7.5
Fe(OH)2 begins to precipitate at pH = 7.5. For best separation, the pH should be as high as possible to precipitate as much Sn(OH)2 as possible, but it must be belowpH = 7.5to avoid precipitating Fe(OH)2. Thus,pH = 7is the optimum pH in the selection.

What are the concentrations of the metal ions in solution after precipitation?
[Fe2+] =
0.01_0__
Fe(OH)2 does not precipitate at pH = 7, so [Fe2+] = 0.01 M
M

[Sn2+] =
1e-14_0__
Use pH = 7 to determine that 
[OH1−] = 1e–07 M,
which is then used in the Ksp expression for Sn(OH)2, [Sn2+] =
= 1e−14 M.
M
| 1e−28 |
| (1e−07)2 |
Note that the precipitated hydroxide could be redissolved in an acidic solution, so the solution of two metal ions could be separated into two solutions that each contain only one metal ion.
8.3-8. Reaction Table Exercise
Exercise: 8.12
Determine the mass of Ag2CrO4 (Ksp = 1.1e–12, Mm = 331.7 g/mol) that is formed and the concentrations of Ag1+ and CrO42– in the resulting solution when 50. mL of 0.10 M K2CrO4 and 50. mL of 0.10 M AgNO3 are mixed.
The equilibrium constant for the precipitation is ~1e+12 (the reciprocal of Ksp because the reaction is the reverse of the dissociation reaction), so essentially all of the limiting reactant disappears.
Fill in the reaction table in millimoles.
The equilibrium constant for the precipitation is ~1e+12 (the reciprocal of Ksp because the reaction is the reverse of the dissociation reaction), so essentially all of the limiting reactant disappears.
Fill in the reaction table in millimoles.
| 2 Ag1+ | + | CrO42– | ⇌ | Ag2CrO4(s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| initial |
5.0_0__
50. mL solution ×
|
5.0_0__
50. mL solution ×
|
0_0__
There is none initially.
|
mmol | ||||||
| Δ |
-5.0_0__
All of the limiting reactant disappears.
|
-2.5_0__
Assume that all of the limiting reactant disappears.
|
o_+2.5_s
Based on the amount of limiting reactant that disappears. Don't forget to include the sign of the change.
|
mmol | ||||||
| equilibrium |
0_0__
Assume that all of the limiting reactant disappears.
|
2.5_0__
Sum the initial and Δ lines.
|
2.5_0__
Sum the initial and Δ lines.
|
mmol |
mass (in grams) of Ag2CrO4 that precipitates =
0.83_0__

2.5 mmol ×
= 8.3e2 mg = 0.83 g
g
| 332 mg |
| mmol |
[CrO42–] =
0.025_0__

[CrO42–] =
= 0.025 M
M
| 2.5 mmol |
| 100 mL |
The stoichiometry of Chapter 1 can be used for all entries except the limiting reactant because the limiting reactant concentration cannot be zero if the Ksp is to be satisfied. Finding the concentration of the limiting reactant is the same as finding the solubility of the salt in the presence of a common ion (the excess reactant). Use the Ksp expression and the [CrO42–] determined above to find the limiting reactant concentration, which will be small but not zero.
[Ag1+] =
6.6e-6_0__

[Ag1+] =
=
= 6.6 × 10−6 M
M
 |
|
 |
|
8.3-9. Reaction Table Exercise
Exercise 8.13:
What is the concentration of lead ions in a solution formed by mixing 24 mL of 0.10 M Pb(NO3)2 and 50. mL of 0.12 M KF?
Be sure to include coefficients with the substances in the balanced equation. (Indicate any subscripted characters with an underscore (_) and any superscripted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.)
Be sure to include coefficients with the substances in the balanced equation. (Indicate any subscripted characters with an underscore (_) and any superscripted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.)
| Cation | Anion | Product | ||||
|---|---|---|---|---|---|---|
|
o_Pb^2+_s
PbF2 precipitates.
|
+ |
o_2F^1-_s
PbF2 precipitates.
|
→ |
o_PbF_2_s
PbF2 precipitates.
|
||
| initial |
2.4_0__
(24 mL)(0.10 M) = 2.4 mmol Pb2+
|
6.0_0_2_
(50 mL)(0.12 M) = 6.0 mmol F1–
|
0_0__
There is no PbF2 initially.
|
mmol | ||
| Δ |
-2.4_0__
Pb2+ is the limiting reactant.
|
-4.8_0__
Pb2+ is the limiting reactant, and 2 mol F1– are required for each 1 mol Pb2+.
|
o_+2.4_s
Pb2+ is the limiting reactant, and one mole of PbF2 forms for each mol of Pb2+ that reacts.
|
mmol | ||
| equilibrium |
0_0__
Sum the initial and Δ lines:
2.4 − 2.4 = 0. All of the limiting reactant disappears.
|
1.2_0__
Sum the initial and Δ lines:
6.0 − 4.8 = 1.2 mmol F1−
|
2.4_0__
Sum the initial and Δ lines:
0 + 2.4 = 2.4 mmol PbF2
|
mmol |
[F1–] =
0.016_0__
[F1–] = 1.2 mmol/74 mL = 0.016 M
M

[Pb2+] =
1.4e-4_0__

[Pb2+] =
=
= 1.4e−04 M
M
| Ksp |
| [F1−]2 |
| 3.7e−8 |
| 0.0162 |
8.4 Complex Ions
Introduction
Complex ions are ions in which a central metal ion is surrounded by molecular or anionic ligands. For example, the [Fe(H2O)6]3+ ion discussed in Section 8.1 is a complex ion. The ligands and the metal are in equilibrium in much the same way that the protons and anion of a polyprotic acid are in equilibrium. That is, there is a series of equilibria in which the ligands are added or removed one at a time. However, in our treatment of complex-ion equilibria, we will consider only the overall process in which all of the ligands are added or removed in one step.Objectives
-
•Write the formation reaction for a given complex ion.
-
•Write the formation constant expression for a given complex ion.
8.4-1. The Formation and Dissociation Constants
The equilibrium constant governing the one-step formation of a complex ion from its metal ion and ligands is called the formation constant, Kf. Table 8.3 contains the formation constants at 25 °C for some common complex ions.| Complex Ion | Kf | Kd |
|---|---|---|
| Ag(NH3)21+ | 1.7e07 | 5.9e–08 |
| Ag(CN)21– | 3.0e20 | |
| Cu(NH3)42+ | 4.8e12 | 2.1e–13 |
| Fe(CN)64– | 1.0e35 | 1.0e–35 |
| Fe(CN)63– | 1.0e42 | 1.0e–42 |
| Ni(NH3)62+ | 5.6e08 | 1.8e–09 |
| Zn(OH)42– | 2.8e15 | 3.6e–16 |
Table 8.3: Formation and Dissociation Constants at 25 °C
As an example, consider formation of the complex ion Ag(NH3)21+.
Ag1+ + 2 NH3 ⇌ Ag(NH3)21+
The equilibrium constant for the reaction is the formation constant of the Ag(NH3)21+ ion.
Kf =
= 1.7 × 107
| [Ag(NH3)21+] |
| [Ag1+][NH3]2 |
8.4-2. Exercise
Exercise 8.14:
What is the concentration of free Cu2+ in a 0.26 M solution of Cu(NH3)42+ (Kd = 2.1 × 10–13)?
Use x for the amount of Cu(NH3)42+ that reacts and determine the equilibrium line for the reaction table.
Use x for the amount of Cu(NH3)42+ that reacts and determine the equilibrium line for the reaction table.
|
Cu(NH3)42+
|
⇌
|
Cu2+
|
+ |
4 NH3
|
||
|---|---|---|---|---|---|---|
| equilibrium |
o_0.26-x_ins
Initial = 0.26;
Δ = −x
|
o_x_ins
Initial = 0;
Δ = +x
|
o_4x_ins
Initial = 0;
Δ = +4x
|
M |
Assume that x is negligible compared to the initial concentration of Cu(NH3)42+ to set up the equilibrium constant expression and solve for x ([Cu2+]).
[Cu2+] =
7.3e-4_0__
If x is negligible in the subtraction, then
So x = [Cu2+] = 7.3e–4 M M
Kd =
=
| [Cu2+][NH3]4 |
| Cu(NH3)42+ |
| (x)(4x)4 |
| 0.26 |
2.1e−13 =
| 256x5 |
| 0.26 |
So x = [Cu2+] = 7.3e–4 M M
8.4-3. Separations with Sulfide Ion
Metal sulfides are very insoluble, so many metals can be separated by adjusting the sulfide ion concentration in solution. Recall from Section 7.4 that the two Ka equilibria of H2S can be combined to eliminate HS1– ion as follows:H2S + 2 H2O ⇌ S2− + 2 H3O1+ K12 =
= K1K2 = 1.3 × 10−20
| [S2−][H3O1+]2 |
| [H2S] |
pH = 5,
the H2S concentration can no longer be assumed to be 0.10 M because the HS1– concentration can no longer be ignored.
8.4-4. Sulfide Separation Exercise
Exercise 8.15:

To what pH should a solution that is 0.010 M in each Fe2+ and Mn2+ be saturated with H2S to achieve an optimum separation of ions?
-
•Ksp(MnS) = 5.6e–16
-
•Ksp(FeS) = 6.3e–18
[S2–] =
5.6e-14_0__
MnS has the higher Ksp, so it is more soluble. Determine the maximum sulfide from the Ksp and the given Mn2+ concentration. 
[S2−] =
=
M
| Ksp |
| [Mn2+] |
| 5.6e−16 |
| 0.010 |
Use the K12 expression in the previous section to determine the [H3O1+] required to deliver the above sulfide
ion concentration.
[H3O1+] =
1.5e-4_0__
Use the [S2–] determined above with the K12 value and the fact that saturated H2S is 0.10 M. 
[H3O1+] =
=
M
 |
|
 |
|
The optimum separation pH to the nearest 0.1 pH units is
pH =
3.8_0__
–log(1.5e–4) = 3.82, so the pH must be kept below 3.82. To the nearest 0.1, that is 3.8.

Assume the pH is adjusted to exactly the above pH; i.e., if the above answer is 4.2, assume a pH of 4.20 and calculate the ion concentrations at this pH.
[S2–] =
5.2e-14_0__
Solve K12 expression for [S2–]. [H3O1+] = 10–3.80 = 1.58e–4 M 
[S2−] =
=
= 5.2e−14
M
| K12[H2S] |
| [H3O1+]2 |
| (1.3e−20)(0.10) |
| (1.58e−4)2 |
[Mn2+] =
0.010_0__
The sulfide concentration is below that required for precipitation of MnS.
M

[Fe2+] =
1.2e-4_0__

[Fe2+] =
=
= 1.2e−04
M
| Ksp |
| [S2−] |
| 6.3e−18 |
| 5.2e−14 |
Is this a good separation?
-
Yes Over 1% of the Fe2+ ions remain in solution with the Mn2+. Such a large fraction is not considered a very good separation.
-
No

8.5 Competing or Simultaneous Equilibria
Introduction
As we have seen, metal ions can be involved in acid-base, solubility, and complex ion equilibria. Thus, several components of a solution containing a metal ion may compete with one another for the metal ion. These competing or simultaneous equilibria are the topic of this section.Objectives
-
•Calculate the solubility of a slightly soluble salt in the presence of a substance that forms a complex ion with the metal.
-
•Write the reaction for the dissociation of a basic salt in acid.
-
•Determine the equilibrium constant for the dissociation of a basic salt in acid.
8.5-1. Silver Chloride Dissolves in Ammonia
Ag1+ ions react with both chloride ions and ammonia molecules, so two competing equilibria are established when AgCl is placed in an aqueous solution of NH3 as both NH3 and Cl1– compete for Ag1+.( Reaction 1 )
AgCl(s) ⇌ Ag1+ + Cl1− Ksp = 1.8 × 10−10
( Reaction 2 )
Ag1+ + 2 NH3 ⇌ Ag(NH3)21+ Kf = 1.7 × 107
( Reaction 3 )
2 NH3 + AgCl(s) ⇌ Ag(NH3)21+ + Cl1−
K = KspKf = (1.8 × 10−10)(1.7 × 107) = 3.1 × 10−3
K = 3.1 × 10−3 =
| [Ag(NH3)21+][Cl1−] |
| [NH3]2 |
8.5-2. AgCl in NH3 Exercise
Exercise 8.16:
What is the solubility of AgCl in 1.0 M NH3?
Use x as the solubility of AgCl (x mol/L of AgCl dissolve) to fill in the reaction table. Note that AgCl is a solid, so there are no entries under it. (K = 3.1 × 10–3 for the reaction below.)
Use x as the solubility of AgCl (x mol/L of AgCl dissolve) to fill in the reaction table. Note that AgCl is a solid, so there are no entries under it. (K = 3.1 × 10–3 for the reaction below.)
| 2 NH3 | + | AgCl(s) | ⇌ |
Ag(NH3)21+
|
+ | Cl1– | ||
|---|---|---|---|---|---|---|---|---|
| initial |
1.0_0_2_
The concentration of ammonia is given in the question.
|
0_0__
There is none initially.
|
0_0__
There is none initially.
|
M | ||||
| Δ |
o_-2x_s
Apply stoichiometry and the fact that x mol/L of AgCl dissolve. Don't forget to include the sign.
|
o_+x_s
Apply stoichiometry and the fact that x mol/L of AgCl dissolve. Don't forget to include the sign.
|
o_+x_s
Apply stoichiometry and the fact that x mol/L of AgCl dissolve. Don't forget to include the sign.
|
M | ||||
| equilibrium |
o_1.0-2x_s
Add the initial and Δ lines.
|
o_x_s
Add the initial and Δ lines.
|
o_x_s
Add the initial and Δ lines.
|
M |
Set up the equilibrium constant expression using the equilibrium line entries. Take the square root of both sides and solve for x, the molar solubility.
solubility of AgCl =
0.050_0_2_
Take the square root of both sides to obtain
Multiply both sides by
Gather terms:

K = 3.1e−3 =
=
| [Ag(NH3)21+][Cl1−] |
| [NH3]2 |
| x2 |
| (1.0 − 2x)2 |
Take the square root of both sides to obtain
0.056 =
.
| x |
| 1.0 − 2x |
Multiply both sides by
1.0 − 2x
to remove the denominator to get 0.056(1.0 − 2x) = x.
Gather terms:
0.056 = 1.11x,
solve for x.x =
= 0.050 M
M
| 0.056 |
| 1.11 |
8.5-3. Acids and Bases Dissolve One Another
The solubility of AgCl is 4 × 103 times greater in 1 M ammonia than in water. In fact, it is a general principle that an insoluble salt can be dissolved by placing it into a solution of a Lewis base (OH1–, CN1–, or NH3) with which the metal forms a complex ion. However, the metal goes into solution predominantly as the complex ion, not as the free metal ion. Dissolving an insoluble salt by forming a complex ion is a Lewis acid-base reaction. Ag1+ is a Lewis acid and Cl1– and NH3 are bases that can compete for the Lewis acid. In cases where the anion is a weak base, the insoluble salt can be dissolved by placing it in strong acid. For example, CN1– is a base and Ag1+ and H3O1+ are acids that can compete for the base. An example of this type is considered in the following exercise.8.5-4. Determining K Exercise
Exercise 8.17:
F1– ion is a weak base that can react with acids. In this exercise, we use the solubility of CaF2 in a strong acid to examine the competition between H3O1+ and Ca2+ for the F1– ion.
What is the equilibrium constant for the dissociation of CaF2 in strong acid?
What is the equilibrium constant for the dissociation of CaF2 in strong acid?
CaF2(s) + 2 H3O1+ ⇌ Ca2+ + 2 HF + 2 H2O
-
•Ksp for CaF2 = 3.9 × 10−11
-
•Ka for HF = 7.2 × 10−4
1. The chemical equation for the Ksp of CaF2 is the following. (Indicate any subscripted characters with an underscore (_) and any superscripted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.)
|
o_CaF_2_s
CaF2 ⇌ Ca2+ + 2F1–
|
⇌ |
o_Ca^2+_s
CaF2 ⇌ Ca2+ + 2F1–
|
+ |
o_2F^1-_s
CaF2 ⇌ Ca2+ + 2F1–
|
2. By what number should the above be multiplied? Use a negative sign to indicate that the direction of the reaction must be reversed from the defined direction.
multiply CaF2 dissociation by
multiply CaF2 dissociation by
1_0__
One mole CaF2 appears as a reactant in both the dissociation reaction and the above reaction, so the reaction does not need to be changed.

3. The equilibrium constant for the reaction produced in Step 2:
K =
3.9e-11_0__
The reaction is the same as the Ksp reaction, so 
K = Ksp.
4. The chemical equation for the Ka of HF is the following. (Indicate any subscripted characters with an underscore (_) and any superscripted characters with a caret (^). For example, NH_4^1+ for NH41+. Include any coefficients other than 1. Omit any spaces.)
|
o_HF_s
HF + H2O ⇌ H3O1+ + F1–
|
+ |
o_H_2O_s
HF + H2O ⇌ H3O1+ + F1–
|
⇌ |
o_H_3O^1+_s
HF + H2O ⇌ H3O1+ + F1–
|
+ |
o_F^1-_s
HF + H2O ⇌ H3O1+ + F1–
|
5. By what number should the above be multiplied? Use a negative sign to indicate that the direction of the reaction must be reversed from the defined direction.
multiply HF dissociation by
multiply HF dissociation by
-2_0__
One mole HF2 is a reactant in the acid dissociation reaction, but two moles of HF are produced in the above reaction. Thus, the Ka reaction must be reversed and multiplied by 2.

6. The equilibrium constant for the reaction produced in Step 5:
K =
1.9e6_0__
Reversing a reaction requires taking the reciprocal of K, and multiplying a reaction by 2 requires squaring K. Thus, 
K = (1/Ka)2.
7. The equilibrium constant for dissolving CaF2 in acid:
K =
7.5e-5_0__
The equilibrium constant is the product of the two equilibrium constants for the reactions that summed to the desired reaction. 
K = (3.9e−11)(1.9e6) = 7.5e−05
8.5-5. Solubility of Ag3PO4
Exercise 8.18:
In Section 8.2-5., we saw that the solubility of Ag3PO4 in water was over eight times greater than that determined with Ksp due to a competition between Ag1+ and H2O for PO43–. We now revisit that solubility as a competing equilibria problem. We assume that the equilibrium can be determined with the following:
Assume that the reaction is Ag3PO4(s) + H2O ⇌ 3 Ag1+ + HPO42– + OH1– and determine the equilibrium constant for the solubility:
Ag3PO4(s) ⇌ 3 Ag1+ + PO43– Ksp = 2.6 × 10–18
PO43– + H2O ⇌ HPO42– + OH1– Kb = 0.021
K =
5.5e-20_0__
Adding the two chemical equations produces the desired equation, so K = KspKb.

If the pH of a saturated solution is 9.00, what is the solubility of Ag3PO4? As shown in Figure 7.3, all phosphate is in the form of HPO42– at pH = 9.00.
solubility =
1.2e-4_0__
K = [Ag1+]3[HPO42–][OH1–];
[Ag1+] = 3x; [HPO42–] = x; [OH1–] = 10–pOH = 10–5.00

The observed solubility is 1.5 × 10–4 M, but that determined with Ksp alone is 1.8 × 10–5 M. Thus, it is competing equilibria like this that lead to such poor agreement between predicted and experimental solubilities for salts of relatively strong bases when only Ksp is used in the calculations.